Translate this page into:
Perioperative outcomes following surgery for brain tumors: Objective assessment and risk factor evaluation
Address for correspondence: Dr. Aliasgar V. Moiyadi, Department of Head Neck Surgery, Neurosurgical Services, Room 48, Main Building, Tata Memorial Hospital, E Borges Road, Parel, Mumbai – 400 012, India. E-mail: aliasgar.moiyadi@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Perioperative outcomes following surgery for brain tumors are an important indicator of the safety as well as efficacy of surgical intervention. Perioperative morbidity not only has implications on direct patient care, but also serves as an indicator of the quality of care provided, and enables objective documentation, for comparision in various clinical trials. We document our experience at a tertiary care referral, a dedicated neuro-oncology center in India.
Materials and Methods:
One hundred and ninety-six patients undergoing various surgeries for intra-axial brain tumors were analyzed. Routine microsurgical techniques and uniform antibiotic policy were used. Navigation/ intraoperative electrophysiological monitoring was not available. The endpoints assessed included immediate postoperative neurological status, neurological outcome at discharge, regional complications, systemic complications, overall morbidity, and mortality. Various risk factors assessed included clinico-epidemiological factors, tumor-related factors, and surgery-related factors. Univariate and multivariate analysis were performed.
Results:
Median age was 38 years. 72% had tumors larger than 4 cm. Neurological morbidity, and regional and systemic complications occurred in 16.8, 17.3, and 10.7%, respectively. Overall, major morbidity occurred in 18% and perioperative mortality rate was 3.6%. Although a few of the known risk factors were found to be significant on univariate analysis, none achieved significance on multivariate analysis.
Conclusions:
Our patients were younger and had larger tumors than are generally reported. Despite the unavailability of advanced intraoperative aids we could achieve acceptable levels of morbidity and mortality. Objective recording of perioperative events is crucial to document outcomes after surgery for brain tumors.
Keywords
Intra-axial brain tumors
neurological outcomes
perioperative outcomes
surgical complications
Introduction
Assessment of outcomes is an integral part of evaluation of any form of therapy. Neuro-oncology has evolved into a specialty in its own right, with dedicated neuro-oncology services providing comprehensive care for patients with brain tumors.[1] Surgery remains the primary (and often only) modality of treatment for brain tumors. On the one hand there is unequivocal evidence of the survival benefit of radical resection.[2–8] On the other hand it is extremely important to preserve and possibly restore neurological function. With advances in imaging technology, many more tumors are being detected earlier at a stage when the patient may be neurologically well preserved. The possible advantages of surgery have to be weighed against the potential risks involved,[2] which are often the limiting factor in radical surgery. Most neurosurgeons today would attest to the principle of ‘safe maximal resection’. Technological adjuncts such as navigation, intraoperative imaging, and intraoperative monitoring equip neurosurgeons to achieve these goals. The goals, when attempting control of the tumor, are twofold — a long-term goal of oncological control (reflected in the progression-free, disease-free, and overall survival); and the short-term goal of ensuring minimal therapy-related toxicity (which in the context of surgery would translate into immediate perioperative outcomes). With a spate of large studies looking at adjuvant therapies in brain tumors, the focus is more on the former goal, and rightly so. However, as local therapy (in the form of surgery) is very crucial in the control of CNS tumors (especially gliomas), systematic and objective documentation of perioperative outcomes is important. Not only does it provide a baseline data for a center, which can be very useful for patient counseling with regard to the risks of surgery (which may differ from center to center), but also allows a particular service / center to objectively assess the benefits of introduction of new technology. Moreover, it provides a yardstick for comparison across various centers, especially when multicentric trials are conducted (in which lack of uniformity and objective comparator indices across centers is usually a significant limitation). This is especially relevant for resource-limited services in developing countries, attempting to balance cost-constrained infrastructure with optimal results. Our department at the Tata Memorial Center, Mumbai, is a dedicated neurosurgical oncology service. This report is an attempt to objectively document the perioperative outcomes after various extirpative surgeries for different intra-axial brain tumors.
Materials and Methods
A prospective database has been maintained of all patients undergoing any form of surgery for brain tumors. For this study only patients undergoing craniotomy for attempted extirpative surgery for intra-axial brain tumors between January 2007 and December 2009 were selected. The study was approved by the institutional review board.
Standard microneurosurgical principles were followed. Intraoperative ultrasound was used whenever deemed required. No other intraoperative adjuncts (navigation, intraoperative monitoring) were available in this time period. Awake craniotomy with clinical monitoring was utilized in select cases. A uniform policy of antibiotic prophylaxis (single dose perioperative second or third generation cephalosporin) was applied. All patients were operated under a perioperative cover of corticosteroids (dexamethasone), which was tapered postoperatively. Antiepileptic medications were used in all patients perioperatively. Non-pharmacological deep venous thrombosis prophylaxis, in the form of intermittent pneumatic compression devices and thrombo-elastic devices (stockings) were utilized with pharmacological prophylaxis (heparin or low-molecular weight heparins) reserved for patients with anticipated prolonged recumbence.
The outcome measures assessed included immediate postoperative (first 24 hours) neurological status, neurological status at discharge, regional complications, systemic complications, overall morbidity, and perioperative mortality. The neurological status at each time point was recorded as same, improved, or worse as compared to the immediate previous assessment. It was further categorized as per severity into minor (minimal alteration of function) or major (significant alteration in function), as well as in terms of duration, as transient (completely or significantly reversible by the time of discharge) or prolonged (minimal or no improvement till the time of discharge). Regional complications included the presence of significant operative site hematoma, worsening or new onset seizures, as well as wound-related complications (which included wound collection, gape, leak, and surgical site infection defined as per the Centers for Disease Control (CDC) criteria[9]). The systemic complications included all other complications such as (but not limited to) metabolic disturbances, hemodynamic complications, systemic infections, and coagulopathy. The overall morbidity (per patient, as one patient could have had more than one of the earlier mentioned complications) as well as mortality (at the time of discharge) were also recorded. For each of the endpoints mentioned various potential risk factors were assessed. These included preoperative predictors (clinico-epidemiological characteristics, such as, age, gender, preoperative neurological status, altered sensorium, KPS score, prior treatment history, comorbid illnesses), surgery-related variables (infra- or supratentorial, emergency surgery, duration of surgery [more than or less than four hours], head shaving, use of wound drain, use of Intraoperative Ultrasound (IOUS), and the extent of resection [subjectively based on surgeon's impression and postoperative CT scans]), as well as tumor-related factors (size [single largest dimension more than or less than 4 cm], location, and histology). This analysis was performed for the whole set of patients as well as for the subset of gliomas (n = 130) as well as glioblastomas (n = 65). However, because of the small numbers, risk factor analysis was carried out in the entire group of intra-axial tumors (n = 196) only.
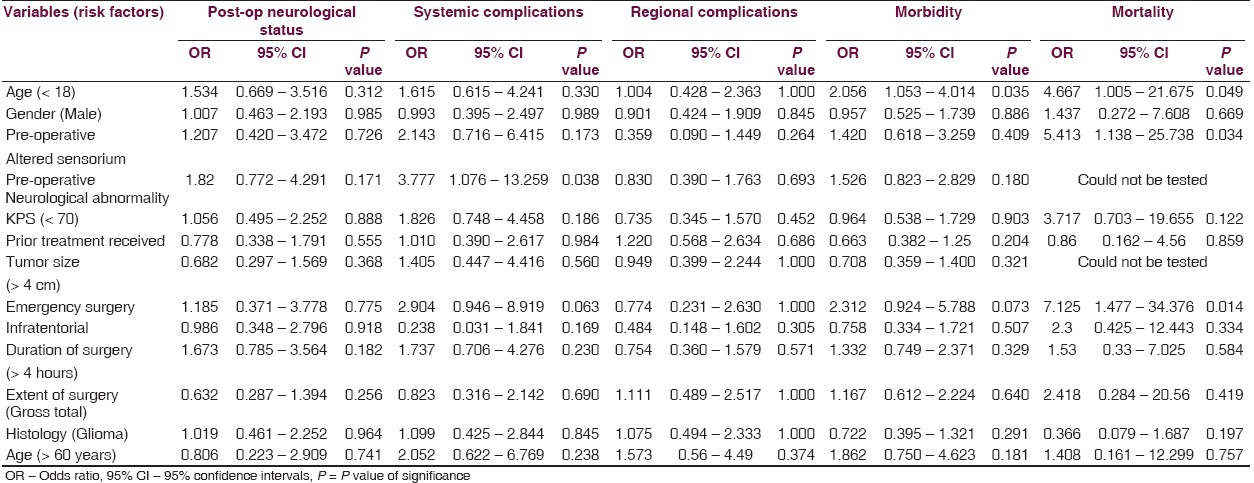
For the purpose of statistical analysis, the risk factors were dichotomized as shown in Table 1. The endpoints were also dichotomized to denote the presence or absence of a particular complication. For neurological outcomes, improvements and no change were considered as favorable outcomes, whereas, worsening was considered as a complication for uni- and multivariate analysis. Univariate analysis was carried out first to determine the association of all risk factors with each outcome. Binary logistic regression analysis using the enter method was used for multivariate analysis. Only those risk factors found significantly (or highly suggestive) associated, or clinically relevant, were included in the multivariate model for risk prediction. Results were tabulated as odds ratio (and adjusted odds ratios for multivariate analysis) with 95% confidence intervals and p values (significant < 0.05). The statistical analysis was performed using SPSS version 15 (SPSS Inc., Chicago, Illinois, USA).
Results
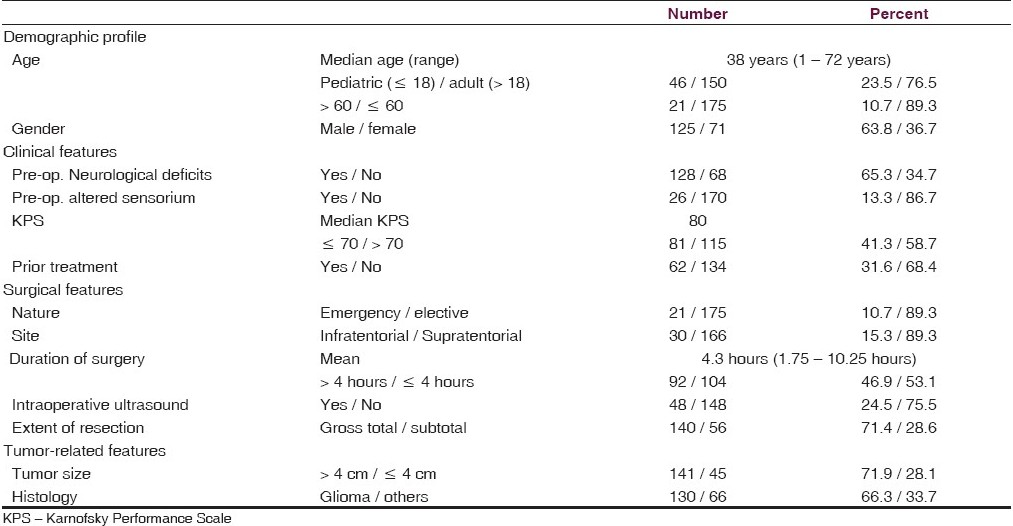
Of a total of 286 patients operated upon in the study period, 23 that did not undergo a craniotomy (stereotactic biopsy, shunt, burr hole) were excluded. Another 67 had extra-axial tumors. Thus 196 patients with intra-axial tumors qualified for this analysis.
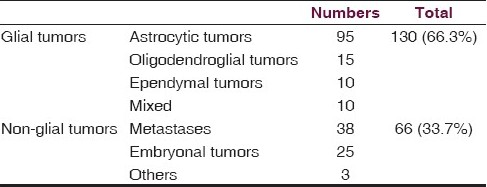
The demographic profile, clinical features, surgical details, and tumor-specific features are detailed in Table 1. The median age of our patient set was 38 years. There were 130 glial tumors (66.3%). The non-glial tumors included 38 metastases (19.4%) and 25 embryonal tumors (12.8%) [Table 2].
Perioperatve complications:
The overall complications encountered are as summarized in Table 3. One hundred and sixty-three patients (83.2%) remained the same neurologically (n = 108 [55%]) or had improved (n = 55 [28%]) postoperatively. Of the 108 who remained the same, only six had improved further till the time of discharge and three had died due to other complications. On the other hand, of the 55 with immediate postoperative improvement, 53 further improved till discharge. Thus, neurological improvement was seen in the immediate postoperative period and was a dynamically sustained phenomenon in those that improved, evolving over the postoperative period. At the same time neurological worsening was encountered in 33 (16.8%), of whom one-third (n=11) had minor deficits (all being transient except one) and two-thirds (n = 22) suffered major deficits (only six improved till discharge). Comparing the postoperative neurological outcomes with the preoperative neurological status revealed that of the 68 (34.7%) preoperative, neurologically normal patients, eight (11.8%) experienced postoperative worsening (three being minor deficits, two of which were transient, and five were major, of which two were transient). There was no mortality in this group. Of the 128 (65.3%) patients with preoperative neurological deficits, 54 (42.5%) remained the same, 46 (38%) had improved, whereas, 25 (19.5%) worsened. All seven patients who died were from this subgroup.
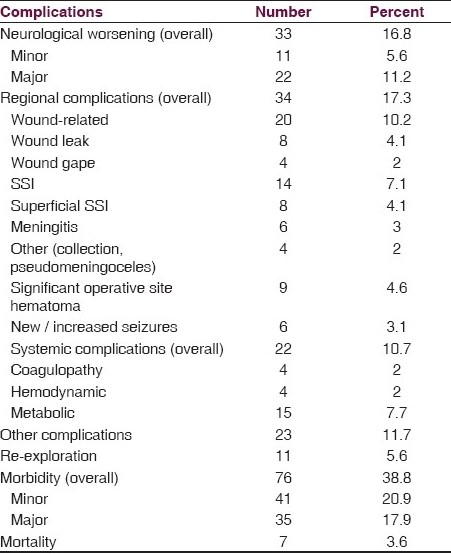
Regional complications were encountered in 17.3% patients [Table 3]. Of these, wound-related complications were the major contributors (10.2%), with surgical site infections predominating (7.1%). Systemic complications occurred in 10.2%, the majority being metabolic disturbances (hyponatremia commonly), most of which were reversible. Clinically significant coagulopathy occurred in only 2%.
Overall morbidity and contribution of neurological morbidity: Although neurological worsening (16.8%) was an important contributor to the overall morbidity (38.8%), it is evident from Table 3 that other postoperative complications (regional and systemic) were significant contributors (if not more) to the overall outcomes. In fact 18.5% of those with no neurological morbidity sustained some other form of morbidity in the postoperative period [Table 4].

The average duration of postoperative stay was 9.2 days (7.2 days for those with no morbidity and 12.4 days for those with some postoperative complication).
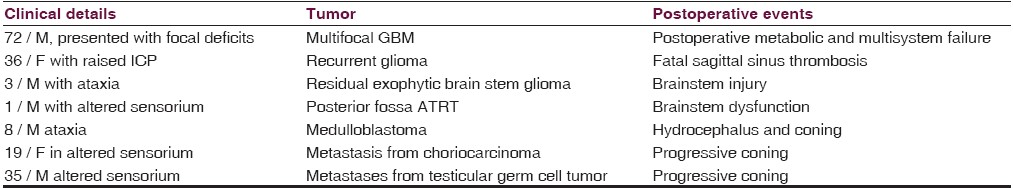
Seven patients died in the postoperative period. The details of the cause of death are depicted in Table 5.
Risk factor assessment
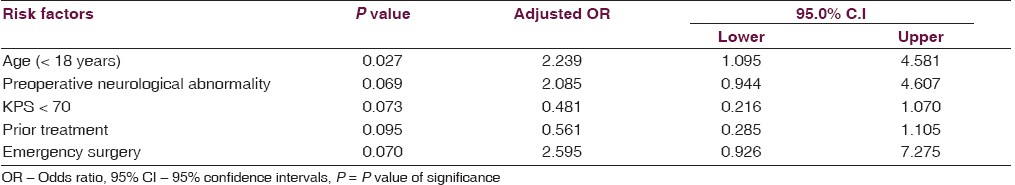
Various risk factors were analyzed for each complication [Table 6]. Binary regression analysis was employed. All the possible factors were initially tested in a univariate analysis [Table 6]. Only the factors found significant were included in the multivariate model [Table 7] for predicting the overall morbidity. On univariate analysis none of the risk factors were significant for immediate postoperative neurological status or regional complications. Patients with preoperative neurological deficits and those undergoing emergency surgery were more likely to have systemic complications. Age less than 18 years and emergency surgery were predictive of increased morbidity and mortality. In addition, the presence of preoperative altered sensorium was predictive of higher perioperative mortality. On multivariate analysis of the risk factors for overall morbidity only age less than 18 years was found significant, although the others did show a trend toward being significant [Table 7].
Discussion
Perioperative outcomes are a measure of the short-term efficacy (neurological improvement and symptomatic relief) as well as the toxicity (perioperative morbidity and mortality) of a surgical intervention. It is indeed very surprising then that more reports dealing with this are not published, as compared to studies dealing with long-term oncological outcomes. With the use of multimodality therapy in the management of brain tumors it is very important to not only separate tumor-related and treatment-related effects, but also to identify the contributions of various treatment modalities toward treatment-related effects. For example neurological deficits (especially cognitive) in patients with supratentorial malignant gliomas could be due to the tumor itself, or as a result of surgery, or a consequence of radiotherapy. Appropriate evaluation at various relevant time points (including baseline) is essential. Moreover, this evaluation needs to be objective and uniformly reproducible.
Early neurosurgeons were more concerned with saving lives and the focus was more on mortality reduction. With refinements in technique and advances in adjuncts, reduction in morbidity started being discussed. Since then, numerous studies have been published for intra-axial tumors.[10–13] There are, however, certain limitations that we would like to discuss.
Study populations have been heterogeneous. Not only the clinical profile, but the tumor characteristics of apparently similar populations can be different [Table 8]. Our patients were significantly younger than those reported. Moreover, we had a much larger proportion of patients with big tumors. This we feel is probably because of the referral pattern at our center, which being a tertiary oncology referral center, drains a very large geographical region encompassing the breadth of the country, including remote and underserved areas, resulting in a significant time lag from the onset of symptoms to access to the necessary facilities and expertise. Although the influence of the preoperative tumor size on long-term outcomes is questionable, there is no doubt that larger tumors present a greater challenge during surgery and can adversely influence the perioperative outcomes. Moreover, it is likely that patients with larger tumors have raised intracranial pressure and altered neurological status, which was our experience too. Although the preoperative neurological status was a significant risk factor for overall morbidity and mortality on univariate analysis, it did not achieve significance on the multivariate model. This could be due to the small size of our series.
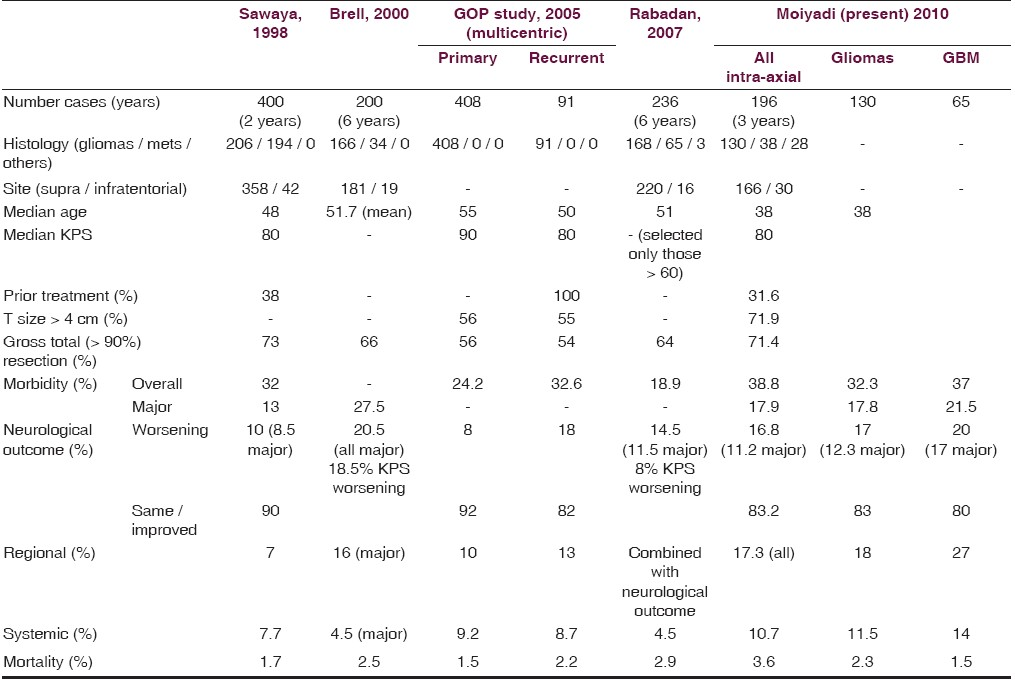
Another problem in comparing with literature is the lack of uniformity in reporting risk factors and end-points. Most studies report the karnofsky performance scale (KPS) as a surrogate marker of clinical status. The KPS, although an objective assessment tool, has significant limitations. It may not reflect all the neurological deficits accurately and may often underestimate the neurological morbidity. Few of the studies have reported neurological worsening, but others have reported only changes in KPS. A small but definite and measurable neurological worsening may not reflect in the KPS. Others have used more specific neurological outcome scales such as the NIH Stroke Score.[14] Nonetheless a scoring system, even as it ensures uniformity in measurement, tends to group outcomes and ignores small (but often clinically significant) differences. On the other hand the recording of individual neurological deficits may overestimate the neurological outcomes (both improvements as well as worsening). This (along with a more detailed reporting of regional complications in our series) may be a reason for the slightly increased overall morbidity we experienced.
For documenting surgical site infection, we rigorously followed the CDC guidelines as part of a parallel prospective study, documenting wound infections in our service. Nonetheless, the major morbidity in our series still compared favorably [Table 8]. We believe that the neurological status more accurately reflected the patients’ clinical status and by meticulously documenting the status we were able to observe the temporal course of these deficits. Our results showed that neurological worsening (new deficits in 11.8% and aggravation of the existing deficits in 19.5%) was a significant issue; however, most of these deficits resolved by the time of discharge. Moreover there was a good chance (almost double, 38%) that the existing neurological deficits would improve. This information was very crucial while counseling and preparing patients for surgery. Moreover, neurosurgeons should be cognizant of the fact that non-neurological morbidity was also a significant cause of postoperative complications.
Another important consideration was the resources available at various treating centers. Most reports were from western and developed nations, with access to the latest adjuncts and fewer cost constraints. During the period of the present analysis, we did not have navigation or intraoperative monitoring available. This was often the case with most resource-constrained centers in developing countries. Even as technological adjuncts definitely increased the surgeon's comfort level during surgeries and possibly allowed more extensive resections to be performed safely, their role in objectively improving the perioperative outcomes remains debatable.[15] Our results showed that even without the use of such aids, acceptable outcomes could be achieved. Being a single center, single surgeon service allowed us to have a uniform perioperative management policy, along with a meticulously maintained, prospective, database-ensured, reliable data capture. However, sustained and regular documentation of such seemingly mundane data was crucial to generalize these results across similar centers across the world.
Limitations of our study: Postoperative MR imaging for documenting residual disease was not always logistically possible, thus limiting objective volumetric assessment in all cases. We hope to address this in our prospective evaluation subsequently. Moreover, although we did attempt to assess individual risk factors, the relatively small size of our study group may not have accurately reflected the true association precluding the statistically significant results.
We would like to acknowledge all the members of the neuro-oncology working group, who were involved at various times in the management of all our patients. We would also like to thank Dr. Rajesh Dikshit and Sadhana Kannan from the Department of Biostatistics and Epidemiology for guidance in the statistical analysis.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- The Emerging Field of Neurosurgical oncology: Novel techniques to optimize outcomes and minimize mishaps. Clin Neurosurg. 2007;54:36-46.
- [Google Scholar]
- Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753-64. discussion 264-6
- [Google Scholar]
- Management of and prognosis with medulloblastoma: Therapy at a crossroads. Arch Neurol. 2008;65:1419-24.
- [Google Scholar]
- The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:33-43.
- [Google Scholar]
- The role of surgery in high-grade glioma--is surgical resection justified? A review of the current knowledge. Ann Acad Med Singapore. 2007;36:358-63.
- [Google Scholar]
- Extent of resection in malignant gliomas: A critical summary. J Neurooncol. 1999;42:303-5.
- [Google Scholar]
- Surgical management of newly diagnosed glioblastoma in adults: Role of cytoreductive surgery. J Neurooncol. 2008;89:271-86.
- [Google Scholar]
- Surgical management of malignant gliomas - Challenges and strategies. Int J Neurol Neurosurg. 2009;1:21-7.
- [Google Scholar]
- Hospital infection control practices advisory Committee (HICPAC) and Centers for Disease Control and Prevention (CDC). Guidelines for prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;24:247-78.
- [Google Scholar]
- Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-55.
- [Google Scholar]
- Factors influencing surgical complications of intra-axial brain tumours. Acta Neurochir (Wien). 2000;142:739-50.
- [Google Scholar]
- Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98:1175-81.
- [Google Scholar]
- Factors related to surgical complications and their impact on the functional status in 236 open surgeries for malignant tumors in a Latino-American hospital. Surg Neurol. 2007;68:412-20. discussion 420
- [Google Scholar]
- Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicenter phase III trial. Lancet Oncol. 2006;7:392-401.
- [Google Scholar]
- Commentary: Surgery for glioblastoma and influence of technology on outcomes between developed and developing parts of the world. Surg Neurol. 2010;73(2):e16.
- [Google Scholar]






