Translate this page into:
Parry-Romberg syndrome with hemimasticatory spasm in pregnancy; A dystonia mimic
Address for correspondence: Dr. Akhila Kumar Panda, Department of Neurology, Institute of Human Behaviour and Allied Sciences (IHBAS), Delhi, Type IV, Q No-12, IHBAS Hospital, Dilshad Garden, Delhi - 110 095, India. E-mail: drakhilapanda@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Parry-Romberg syndrome (PRS) with hemimasticatory spasm (HMS) is quite an uncommon overlapping phenomenon which very often mimics jaw closing dystonia. A previously healthy 35-year-old female, during her 5th month of pregnancy started developing intermittent unilateral painful spasms of jaw while conversation, clinching of teeth, or eating, which led to frequent tongue bites. The spasms were worsened during pregnancy. She used to do certain manoeuvre like sensory tricks in form of touching involved side of the face to relieve the symptoms. Apart from this, she developed progressive hemifacial and hemitongue atrophy. Other medical and neurological examinations were normal. Laboratory investigations as well as neuroimaging were noncontributory. The spasm responded to carbamazepine but hemifacial atrophy persists. To our best knowledge, onset and worsening of this syndrome in pregnancy has not been described earlier which might be correlated either with some hormonal imbalance or some unknown mechanisms.
Keywords
Hemimasticatory spasm
hemifacial spasm
jaw dystonia
Parry-Romberg syndrome
Introduction
Parry-Romberg syndrome (PRS) is an uncommon acquired neurological disorder which includes progressive hemifacial atrophy involving the fat, skin, connective tissue, and sometimes bone.[1] The severity ranges from mild asymmetry to severe disfigurement. PRS may be overlapped with various clinical entities like tongue atrophy, hemimasticatory spasm (HMS), linear scleroderma, epilepsy, migraine, facial pain, and dermatologic manifestations like vitiligo and hyperpigmentations.[1] Since the first description by Caleb Parry (in 1815) and by Moritz Romberg (in 1846), several anecdotal case reports have been subsequently documented.[1] PRS and HMS are known to co-occur which affects unilateral muscles of mastication.
Case Report
A 35-year-old female, mother of single child, has been experiencing intermittent involuntary painful spasms and locking of jaw for 2-3 min, resulting in frequent tongue bites. This symptom developed during her 5th month of pregnancy and dramatically worsened in subsequent gestation period. The initial frequencies in 5th and 6th months of pregnancy were 2-3 episodes per day and subsequently increased to 6-8 times per day. The intensity of spasms was also severe for which the patient was socially embarrassed and occasionally cried during those periods. One month after delivery, the spasms began to diminish rapidly and progressively dropping to lesser intensity of brief spasm of frequency 2-3 episodes per day which persisted till next 2 year when she consulted us and get into hospitalization.
She frequently developed spasms while eating, laughing, and sometimes even during normal conversation. The spasms persisted during sleep, disturbing, and sometimes awakened her from it. During the spasm, she used to do certain manoeuvre like rubbing her hand to the left face and tried to open her jaw to get rid of it. She denied dysarthria, dysphagia, or breathing difficulty. There was no associated dystonia, tremor, myoclonus, or other involuntary movements. After 4 months of onset of the above symptoms, she noticed progressive thinning of the left half of face including the tongue.
General physical examination was unremarkable. Neurological examination disclosed marked wasting of left side of the face over the cheek, mandible, and temple [Figure 1]. There was no weakness of face or muscles of mastication. Left half of the tongue was atrophied without any weakness, fasciculations, or impairment of taste sensation [Figure 2]. The right face was spared. Jaw jerk was absent. Sensory examination of face including corneal reflex was normal. Gag reflex and palatal movement on phonation were normal. She did not have hyperpigmentation, depigmentation, skin thickening, or tightness of the face or body. Rest of neurological examination was normal. Examinations of cardiovascular, respiratory, gastrointestinal, or musculoskeletal system were noncontributory. Family history was insignificant.
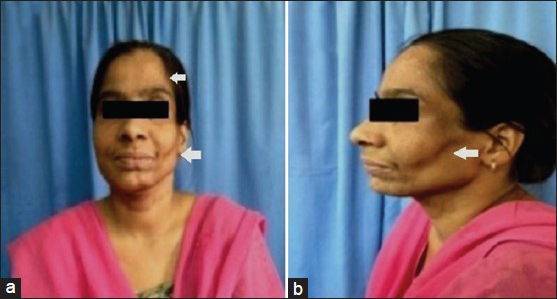
- (a and b) Left hemifacial atrophy involving cheek, masseter, and temple (arrow)
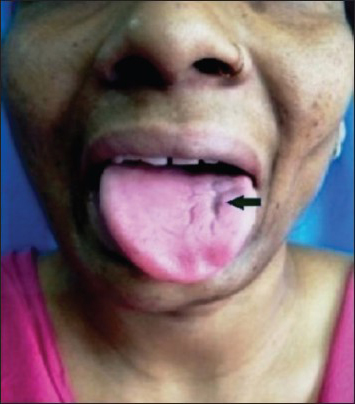
- Atrophy of left half of tongue (arrow)
Routine blood investigations were normal. Markers for human immunodeficiency virus, hepatitis B and C virus and syphilis were negative. Magnetic Resonance Imaging of brain and magnetic resonance angiogram of intracranial vessels were normal. Vasculitic profiles including rheumatoid factor, antinuclear antibody, anti-dsDNA antibody, anti-Scl70 antibody, anti-topoisomerase, anti-centromere antibody, anti-histone antibody were negative. Electroencephalogram, blink reflex, and facial nerve conduction study were normal. Needle electromyography of bilateral facial muscles including massater, temporalis, and mentalis was performed. Spontaneous activities such as high frequency complex repitive discharges were found in left masseter and temporalis muscle [Figure 3] with normal motor unit potentials. Skin biopsy could not be performed due to unwillingness by the patient.
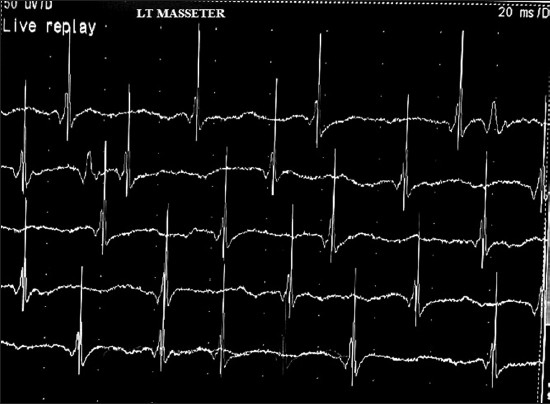
- Needle electromyography of left masseter showing spontaneous high frequency complex repetitive discharges with normal motor unit action potentials even without spasm
She responded partially to carbamazepine (up to 800 mg/day). The spasm frequency decreased from several times a day to once every week. Due to financial constraints, she denied injection botulinum toxin or surgical intervention. We deferred immunotherapy because of lack of evidence of scleroderma or associated cerebral findings as well as the stable course of the disease.
Differential diagnosis
HMS mimics other form of focal facial dystonia such as trismus, hemifacial spasm (HFS), jaw closing dystonia, myotonia, and tetanic spasm. The clinical features of our patient showed the classical description of HMS. However, due to the presence of progressive hemifacial and tongue atrophy, the possibility of PRS along with HMS was considered. In relation to HMS, HFS and unilateral focal jaw closing dystonia were two closest possibilities. In HFS, the initial site of onset is orbicularis oculi muscle (90%), the cheek (11%), and the perioral region (10%).[2] Moreover, there was associated hemifacial atrophy which could not be explained by HFS alone. In focal jaw-closing dystonia, there has been unilateral jaw deviation which occurs episodically and lasts from several days to weeks at a time before remitting, only to be followed by another episode after a variable period of time. HMS do not show co-contraction of antagonists and agonists muscles acting on the jaw as seen in unilateral jaw dystonia.[3]
Discussion
PRS in combination with HMS is a neurological rarity. Most cases of PRS are sporadic with rare exception, where familial instances are documented without specific inheritance pattern.[4] The prevalence of PRS is 1 in 7, 00,000 population with female predominance and usually occurred in first two decade.[5] Late age of presentation has been rarely reported.[6] PRS is very often associated with localized scleroderma called “en coupe de sabre.”[1] PRS may be associated with tongue atrophy (25%) and HMS (35%) as in our case.[1] The spasm usually involves one or more jaw closing muscles (massetor, temporalis, and medial pterygoid) on one side. Though the obvious pathophysiology is not known, still some hypothesis was proposed by various authors both in PRS and HMS. The excitatory electrical activity of trigeminal motor root or the motor nucleus may play a role in generation of HMS.[7] The cramps like activity in HMS may be responsible for high frequency spontaneous discharges and synchronization of muscles due to lateral spread of electrical activity to adjacent nerves. Unlike HFS there is no ectatic vascular compression identified in HMS with hemifacial atrophy.[8] Progressive hemifacial atrophy may be related to trigeminal neurovasculitis secondary to cell-mediated immunity, central sympathetic dysregulation, and associated autoimmune pathology.[9]
In the present case, the symptoms were started during 5th month of pregnancy and worsened in subsequent period of gestation. The spasms were dramatically improved after child birth. The symptoms variability look like the probable correlation between pregnancy-related hormonal imbalance and HMS. Cersósimo et al.,[10] also observed the similar findings in their report.
Hemifacial atrophy in PRS is an irreversible phenomenomenon. Progressive disease with linear scleroderma may be treated with topical steroids, ultraviolet A irradiation, immune suppressant (methotrexate, azathioprine) to halt the disease progression.[1] PRS with cerebral involvement may be treated with immunosuppressive drugs.[11] In “burnt out” case, various aesthetic procedures have been employed. Local injection of calcium hydroxyl apatite has been shown to be helpful in patients with hemifacial atrophy.[12] Autologous fat cell transplantation has been found to be useful in long-term corrections of facial atrophy.[13] Treatment of HMS is challenging. Pharmacotherapy includes carbamazepine, phenytoin with variable results. Botulinum toxin injection into the masticatory muscles is the treatment of choice in HMS.[14]
Our patient had no history of migraine, epilepsy, facial pain, systemic, or localized scleroderma. We highlight some unique features in the present case, (1) The late age of presentation (onset at 33 years), (2) onset and worsening during pregnancy, (3) presence of sensory trick (rubbing the affected side of the face to relieve the symptoms) very often confused jaw closing dystonia, and (4) relatively good response to carbamazepine unlike other series.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Parry-Romberg syndrome: A global survey of 205 patients using the Internet. Neurology. 2003;61:674-6.
- [Google Scholar]
- Central sympathetic dysregulation and immunological abnormalities in a case of progressive facial hemi atrophy (Parry-Romberg disease) Clin Auton Res. 1995;5:199-204.
- [Google Scholar]
- Focal dystonia of the jaw and the differential diagnosis of unilateral jaw and masticatory spasm. J Neurol Neurosurg Psychiatry. 1986;49:651-6.
- [Google Scholar]
- Favorable longitudinal outcome in a patient with Parry-Romberg syndrome. Acta Neurol Scand. 2005;112:192-3.
- [Google Scholar]
- What is your diagnosis? A case of facial atrophy of one half with depression in skin over forehead. Indian J Rheumatol. 2011;6:201-3.
- [Google Scholar]
- Hemimasticatory spasm: Clinical and electrophysiologic observations. Neurology. 1992;42:2263-6.
- [Google Scholar]
- Pathophysiology of hemimasticatory spasm. J Neurol Neurosurg Psychiatry. 1994;57:43-50.
- [Google Scholar]
- Clinical and ultrastructural studies of Romberg's hemifacial atrophy. Plast Reconstr Surg. 1990;85:669-74.
- [Google Scholar]
- Botulinum toxin in a case of hemimasticatory spasm with severe worsening during pregnancy. Clin Neuropharmacol. 2004;27:6-8.
- [Google Scholar]
- Idiopathic hemifacial atrophy treated with serial injections of calcium hydroxylapatite. Dermatol Surg. 2010;36:542-5.
- [Google Scholar]
- Autologous fat transplantation for depressed linear scleroderma-induced facial atrophic scars. Dermatol Surg. 2008;34:1659-65.
- [Google Scholar]
- Hemimasticatory spasm in hemifacial atrophy: Diagnostic and therapeutic aspects in two patients. Mov Disord. 1995;10:504-7.
- [Google Scholar]






