Translate this page into:
Outcome of Early versus Delayed Evacuation of Spontaneous Lobar Hematomas in Unconscious Adults
Address for correspondence: Dr. Wael K. Zakaria, Department of Neurosurgery, Mansoura University Hospital, Gomhorria Street, Mansoura, Egypt. E-mail: drwaelmusa@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To explore the difference in outcomes of medium-sized lobar hematomas evacuated in early versus delayed fashion among unconscious noncomatose individuals.
Methods:
A retrospective analysis of demographic, clinical, and radiological data of unconscious patients admitted with lobar hematomas during 18 years was performed. Time to surgery was compared in various patient variables and characteristics. Outcome groups (favorable and poor) were also compared to find out any association with surgery timing, as well as potential indicators of outcome and mortality.
Results:
The mean follow-up period in this study was 7.5 months after discharge. Two-thirds of the patients carried favorable prognosis at final follow-up with mortality (7.3%) included among poor cases. Time to surgery was not associated to any of the patient characteristics, except for international normalized ratio and associated chest problems which represented the main indicators of delayed surgery. Rebleeding after evacuation was associated with shorter time to surgery in clots ≤35 cc but not in the whole group. Poor outcome was significantly associated with higher basal glucose levels, bigger hematomas, rebleeding after surgery, and delayed evacuation of clots >35 cc. The presence of mild intraventricular hemorrhage (IVH) per se was not associated with increased mortality or poor outcome; however, its volume was.
Conclusion:
Smaller lobar hematomas (≤35 cc) in unconscious adults (Glasgow Coma Scale 8–13) may be managed with initial conservative treatment, while larger hematomas (>35 cc) are better evacuated as early as possible. Basal glucose levels and volume of mild IVH should be considered in the future management planes.
Keywords
Delayed
early
hematoma
lobar
unconscious
INTRODUCTION
Primary intracerebral hematomas (ICHs) account for 10% of all first-ever strokes versus 81% accounted for by cerebral infarctions. However, the mortality rate in primary ICH is fivefold higher at 1 month and about threefold higher at 1 year poststroke as compared to cerebral infarction.[1] This makes primary ICH a neurological emergency with high incidence of fatality being responsible for more than one million annual deaths worldwide.[234] Unfortunately, the best care for such pathological entity is not yet well-defined. Whether to offer surgical or conservative treatment and whether to operate early versus delayed surgical intervention remain unsolved issues.[56789101112131415] This controversy continues because these studies included heterogeneous groups of patients as regards Glasgow Coma Scale (GCS) scores, hematoma volumes, intraventricular extension, and hematoma locations, including basal ganglionic clots, which generally carry more surgical risks and worse outcome, especially with increased perifocal edema.[16] Even more, these studies implemented different surgical approaches with subsequently different approach-related damages.[61115] All these factors impacted the results even in randomized trials. Better and more consistent results can be fulfilled by comparing patients with ICHs of similar types and locations, presented with comparable GCS scores, and operated using similar techniques. The aim of this study was to explore the outcome and mortality differences in a specific lobar hematoma patient subpopulation, who presented unconscious and managed at various timing based on surgeon's perspective and/or patient's status.
METHODS
Patients
This is a retrospective analysis of data records of patients admitted to the Department of Neurosurgery with the diagnosis of spontaneous lobar ICH, during an interval of 18 years from January 1998 to December 2015. Patients included those admitted initially to the Neurosurgical Department in addition to those referred to it from the Neurology Department with the same diagnosis for surgical intervention. Patients included were those presented in a disturbed conscious status yet not comatose (GCS >8 and <14). The included cases were operated according to different timing strategies based on surgeons’ experience and patients’ suitability. Time to surgery was calculated for all patients using the earliest documented point of time available which was the time of medical presentation in the emergency form. Reference to the presentation time was used because data about time of onset of symptoms were missing in a significant proportion of patients to be reliably imputed by imputation methods. The results were thus expected to reflect the effects of the time window from medical presentation rather than from the ictus. The following patients were excluded: (1) those referred from the Department of Neurology in a poor neurological status (derived GCS score ≤8), (2) hematomas secondary to underlying lesions such as vascular malformation, tumor, and venous sinus thrombosis, (3) history of previous stroke, (4) sizable intraventricular clot occupying >1/3 of either lateral ventricle (allowing for partial or complete occlusion of third and fourth ventricles so long as no ventricular expansion detected), (5) uncorrected coagulopathy before surgery, (6) cases with successful conservative treatment and improvement of neurological status, and (7) associated hydrocephalus due to either; the hematoma mass effect or a blood clot obstructing cerebrospinal fluid pathways.
Data collection
The process of data collection was done in two parallel independent processes. It started by reviewing the outpatient clinic follow-up database to select cases with the diagnosis of lobar ICH, who underwent surgical evacuation in the Neurosurgical Department during the mentioned time interval. This was done by one of the authors who collected the serial numbers and follow-up data. The other authors blinded to the follow-up data used the serial numbers of the selected patients to review the department archive and extract the demographic, clinical, radiological, and management data to complete the process of selection by applying the remaining inclusion and exclusion criteria. After completion of data collection, authors provided the data to an independent biostatistician who assembled the data using the serial numbers to start the statistical analysis.
Demographic data included age, sex, marital status, residence, and smoking. Clinical data included initial and immediate preoperative GCS and National Institutes of Health Stroke Scale (NIHSS) scores, in addition to neurological deficits, systolic, diastolic, and mean blood pressures, associated medical conditions such as history of hypertension, diabetes mellitus, liver or renal disorders, and antiplatelet and/or anticoagulant medications. In addition, laboratory tests such as serum glucose and international normalized ratio (INR) were collected for analysis. The level of consciousness in this study was not based on “directly calculated” GCS scores because verbal responses were missing in a significant proportion of patients who presented with aphasia due to hematomas affecting their dominant hemispheres. Instead, we depended on the motor and eye scores to predict the missing verbal response and obtain “derived GCS scores” using a validated regression equation,[1718] then rounding the scores to the nearest integer.
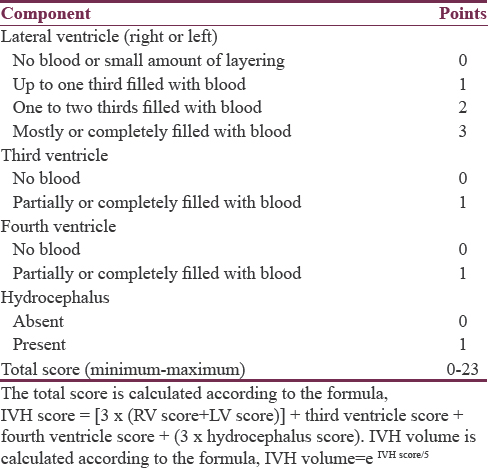
Radiological data included side of hematoma, ICH volume in initial and follow-up computed tomography (CT) images using Broderick's formula (A × B × C/2),[4] minimum distance of ICH from cortical surface (in millimeters), associated intraventricular hemorrhage (IVH) and IVH score according to Hallevi et al. [Table 1], with estimation of the IVH volume and total volume (TV) (TV = ICH volume + IVH volume).[19] Rebleeding was defined as a hematoma size in a follow-up CT >33% of the ICH volume on the previously done CT.[202122] Data related to management including medical treatment, time of intervention, surgical technique, overall hospital stay, and postoperative mortality were also collected for analysis.
Outcome assessment
Functional assessment was performed using the modified Rankin Scale (mRS). The mRS scores of patients were collected at all available points of time from discharge till data collection. The mRS score at the last visit was considered as the final outcome. The final scores were dichotomized into favorable versus poor outcomes taking into account the baseline severity. First, the prognostic scores of patients were calculated from the following equation: 10 × GCS − (age in years) − (0.64 × volume in mL),[15] followed by dividing the patients at the median score into good and poor prognosis groups. For the good prognosis group, favorable outcome was defined as good recovery or moderate disability (mRS ≤3), while for the poor prognosis group, it included also the moderately severe disability (mRS ≤4). Several researchers have proposed this prognosis-based methodology.[2324]
Ethical approval
Written informed consents were provided by relevant individuals before any surgical intervention. The study was assessed and approved by the local committee for medical research, MFM-IRB (code: R/16.03.30).
Missing data and statistical analysis
For statistical analysis, we used the Statistical Package for the Social Sciences (SPSS Statistics) for Microsoft Windows (Version 17.0, 2008; SPSS Inc., Chicago, IL, USA). There was an overall 4.9% missed values in collected data, on which multiple imputation procedure was conducted. The missing pattern was completely at random, the missed values were imputed multiply, the analyses were performed, and the results were pooled into one estimate. Descriptive analyses included mean ± standard deviation (SD) for parametric continuous data and mean and interquartile range (IQR) to describe nonparametric continuous data, while numbers and proportions were used for description of categorical data. For better statistical analysis, we used binary outcomes (dead vs. alive and favorable vs. poor). Comparing the outcome groups was done using Mann–Whitney U-test for continuous nonparametric variables and Chi-square test for categorical data. Significance was set at a P < 0.05.
RESULTS
Eighty-two patients with the diagnoses of supratentorial lobar ICHs were included in the study after fulfilling the inclusion and exclusion criteria.
Demographic data
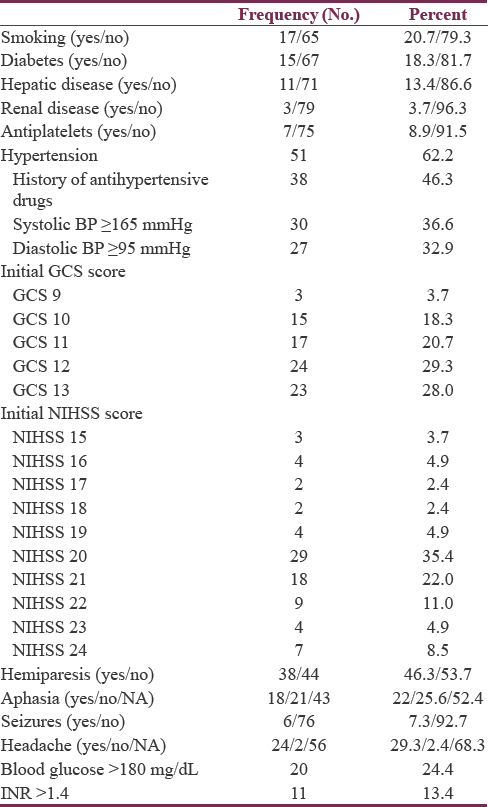
Ages ranged from 55 to 79 years with a mean of 65.31 ± 5.95 years. Among the 82 patients, the majority were females (47 cases, 57.3%). Although history of hypertension was confirmed in only 38 patients, hypertension was diagnosed in 51 cases (62.2%). Diagnosis of hypertension was given for those already on antihypertensive drugs before admission, in addition to those with persistent elevation of systolic (above 165 mmHg) and/or diastolic (above 95 mmHg) pressures requiring continuation of antihypertensive therapy during the whole hospital stay and beyond. The mean was 157 ± 23 mmHg for systolic, 91 ± 10 mmHg for diastolic, and 107 ± 14 for mean blood pressures. Fifty-one cases (62.2%) were rural inhabitants and 68 patients (82.9%) were married.
Clinical presentation
Initial GCS scores ranged from 9 to 13, with a mean of 11.4 ± 1.5. The initial NIHSS ranged from 15 to 24 with a mean of 20.4 ± 2.1, indicating severe impairment. Preoperative GCS scores ranged from 9 to 13 with a mean score of 11.1 ± 1.4, while the corresponding NIHSS scores ranged between 16 and 24 with mean of 21.0 ± 1.9. Neurological deficits detected included hemiparesis and aphasia. Medical history and clinical presentations in patient population are reported in Table 2.
Blood glucose and international normalized ratio
Blood glucose level ranged from 90 to 390 mg/dL (5–22 mmol/L) with a median of 130 mg/dL and IQR of 64 (110–174). About 76% of patients had blood glucose levels below 180 mg/dL and needed no insulin therapy, while the remaining patients (with blood glucose level ≥180 mg/dL) followed a sliding insulin scale. The INR ranged from 1.00 to 2.20 with a mean of 1.24 and SD of 0.29. Those with INR >1.4 needed correction of their coagulation profile before surgical intervention [Table 2].
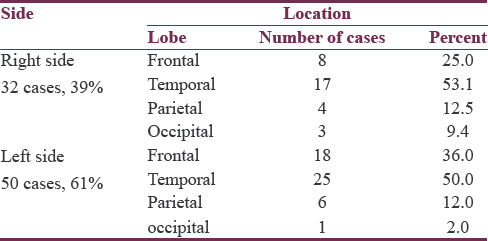
Hematoma location and size
The majority of hematomas (61%) were located on the left side. The most common location was the temporal lobe, followed by frontal, parietal, and then occipital lobes. This pattern of descending frequency was noticed in hematomas of both sides, as well as on each separate side [Table 3]. The size of hematomas ranged from 30 to 52 cc with a mean of 36.8 ± 5.6, median of 35.5, and IQR of 9. Half of hematomas were ≤35 cc. All hematomas were not <10 mm away from the cortical surface, ranging from 0 to 10 mm with a median of 5 mm and an IQR of 5 mm. Of all lobar hematomas, 35 cases (42.7%) were associated with IVH. The IVH scores ranged from 3 to 8 which corresponds to IVH volumes of 2–5 cc, giving TVs of hemorrhages (ICH volume + IVH volume) ranging between 30 and 57 cc.
Intervention time
The median time from presentation to skin incision was 13 h and 55 min, with IQR of 22 h and 40 min for the whole patient population. The time window ranged from 2 h and 40 min in the earliest operated case to 70 h and 30 min in the most delayed one. Among those patients, an initial decision of surgical evacuation was taken in only 33 cases, while in others, surgical evacuation was done after an initial conservative management. Cases with initial decision of conservative treatment included those in whom conservative management was considered promising by the treating neurologist (11 cases), as well as those in whom an associated comorbidity required an initial management to lessen surgical risk (38 cases). Those 49 cases received the best medical management till their transfer to surgical evacuation. Cases whose medical problems became resolved were directly operated, while those who underwent an initial trial of conservative treatment were referred to surgical intervention due to either a drop in their GCS score (1 point at least), a drop in their NIHSS scores (2 points at least), or no improvement.
Management of cases
All patients received the best medical treatment including osmotherapy, antiepileptic drugs, prophylaxis against deep venous thrombosis, H2 blockers, intravenous fluids, maintenance of normoglycemia, fever control, and early nutritional support. All patients were operated on using an open craniotomy (about 5–7 cm in diameter) through a C-shaped scalp incision centered over the most superficial point of ICH. After performing the cortical incision, effective debulking of the hematoma was carried on till the surrounding brain tissue became grossly lax and the cortical surface dropped away from the dura. Achievement of adequate decompression and hemostasis under illumination and magnification of the operating microscope was done using the usual microsurgical equipment and techniques. This was followed by proper closure of the dura with repositioning of the bone flap. Immediate postoperative CT scans revealed residuals ranging from traces to about 10 cc.
Mortality and outcome
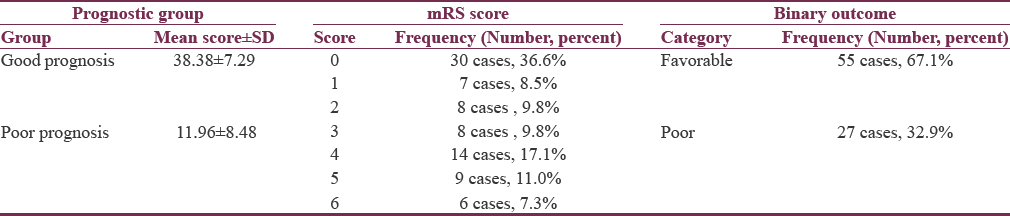
The outcome was assessed using mRS scores. mRS score of six represented dead cases (6, 7.3%) while score 0 represented those with no residual symptoms (30, 36.6%). The last follow up at which outcomes were registered ranged from 2 to 14 months with a mean follow up of 7.5 ± 3 months. About two-thirds of cases were reported to have a favorable outcome at their last follow-up, while the other third of patients including cases of mortality were reported as poor outcome. The final dichotomized outcome was defined based on the initial basal status and predicted prognosis as previously mentioned [Table 4].
Exploring variables to intervention groups
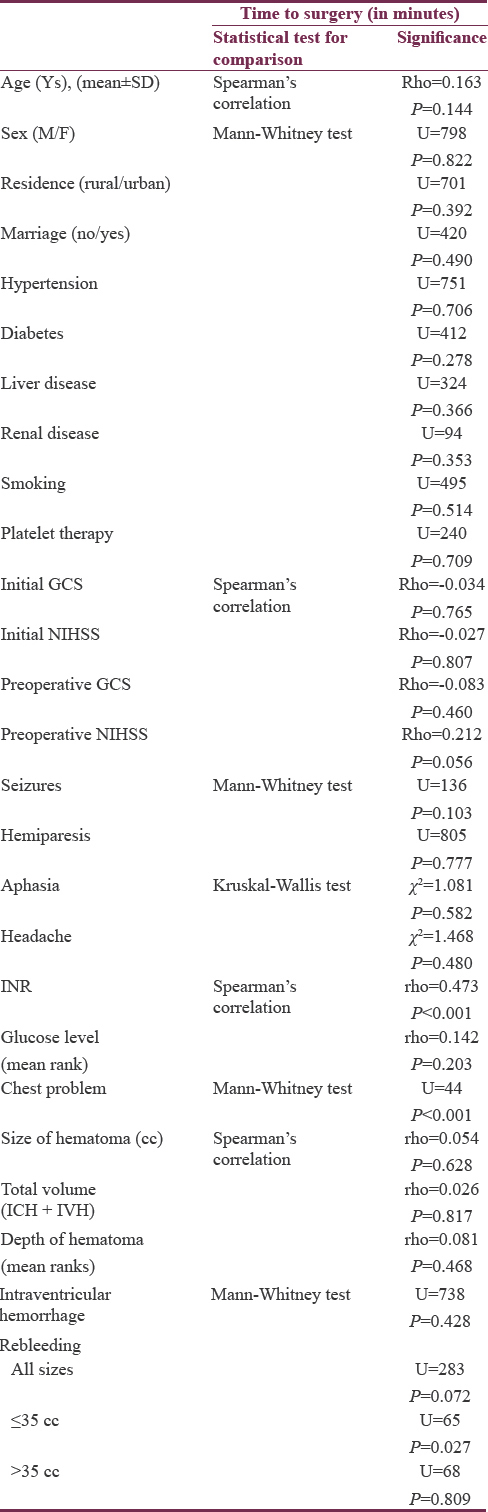
Comparing time of intervention among various patient characteristics revealed no significant association related to age, sex, residence, marriage, hypertension, diabetes and associated chronic diseases, smoking, or use of antiplatelet therapy. The initial GCS and NIHSS scores, in addition to, immediate preoperative GCS scores, also showed no significant association with time of intervention. However, immediately preoperative NIHSS scores showed borderline significant (P = 0.056) weak association (ρ = 0.212) with time of intervention, indicating low probability of new deficits development in delayed surgery. Clinical presentation, glucose level, size and depth of the hematomas, as well as the presence of IVH also failed to show significant association with timing of surgery. Rebleeding was not reported before surgical interventions in both groups but detected after surgical evacuation in 12 cases (14.6%). Although the time of surgery was not found to be significantly different among those who bled and those who did not in the whole group, it was significantly shorter among rebleeding group in hematomas ≤35 cc but not in hematomas larger than 35 cc. The INR and presence of associated chest problems were significantly associated with longer time to surgery, indicating their important role in initial decisions of conservative management. Other variables were not affected by or played a role in the decision of timing of surgery [Table 5].
Exploring variables to outcome
Demographic and clinical data
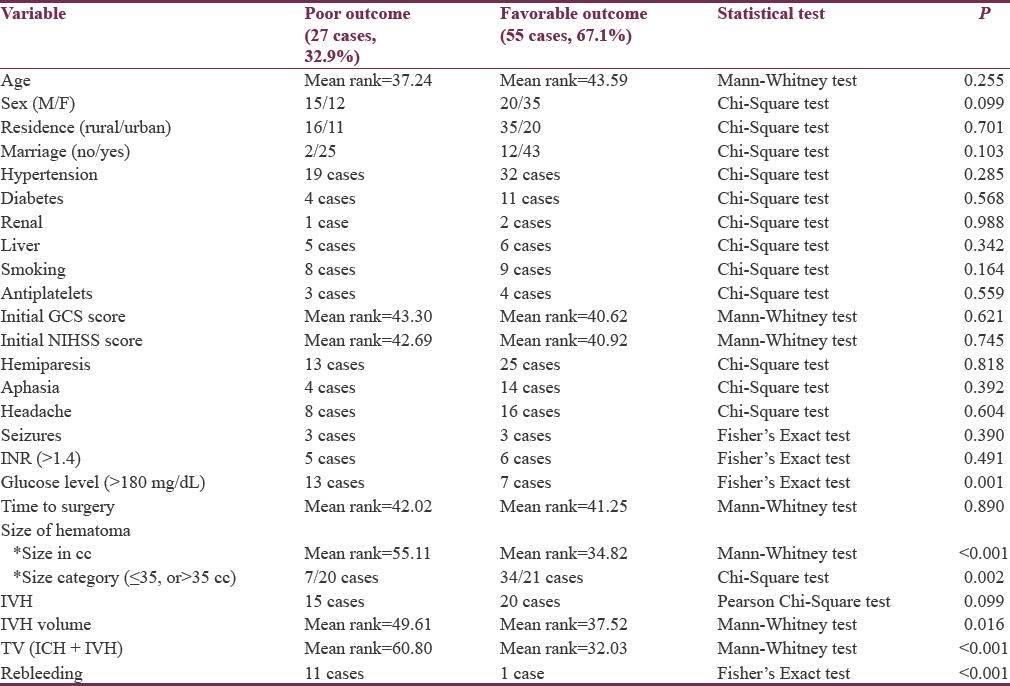
Bivariate analysis of various variables with final outcome (poor vs. favorable) revealed no statistical difference of age, sex, smoking, residence, marriage, associated conditions such as hypertension, diabetes, hepatic, renal and coagulopathy, or use of antiplatelet therapy among outcome groups. Moreover, clinical presentation such as the presenting GCS and NIHSS scores, in addition to neurological deficits, failed to show any significant differences between final outcome groups. On the other hand, serum glucose levels at presentation differed among outcome groups. Glucose levels above 180 mg/dL were significantly more associated with poor outcome [Table 6].
Intracerebral hematomas volume/intraventricular hemorrhage and intraventricular hemorrhage volume/total volume
The mean ICH volume was 40.37 ± 6.46 in the poor outcome and 35.01 ± 4.23 in the favorable outcome groups. The mean TV was 43.55 ± 5.21 and 36.52 ± 4.65, respectively. These figures were significantly reflecting the influence of initial ICH size and total hemorrhage size on final outcome. Although the presence of IVH did not differ significantly between outcome groups, the IVH volume was significantly higher among patients with poor outcome [Table 6].
Intervention time
Comparing the time to surgery in different mRS score categories, as well as in both outcome groups, revealed no significant difference. Data were then split at the median size into two groups (≤35 cc and >35 cc). There were 41 cases with hematoma size ≤35 cc and another 41 hematomas >35 cc. In hematomas ≤35 cc, the time to surgery was shorter in the poor than favorable group; however, this difference failed to reach the statistical significance. On the other hand, in hematomas larger than 35 cc, the poor outcome group showed statistically significant longer time to surgery when compared to favorable group. Previous results suggest that delayed surgery is relatively a safer approach in hematomas ≤35 cc but a poor prognostic indicator in hematomas >35 cc. Receiver operating characteristic (ROC) curve analysis was done using the dichotomized outcome as the state variable and time to surgery as the test variable for the whole population, as well as for those with hematomas larger than 35 cc. Area under the ROC curve when the whole population was analyzed was 0.542, with standard error (SE) = 0.069 and 95% confidence interval (CI) = 0.407–0.678, indicating that the discriminative power of time to surgery in predicting outcome in the whole group was no better than chance. On the other hand, the ROC curve analysis on the subpopulation with hematomas >35 cc was of significant discriminative value (area = 0.732, SE = 0.079, and 95% CI = 0.577–0.888) [Figure 1]. Youden's J statistic was used to select the optimum cutoff point of time to surgery that can predict poor outcome (cutoff 11 h and 15 min with sensitivity of 88% and specificity of 56%).
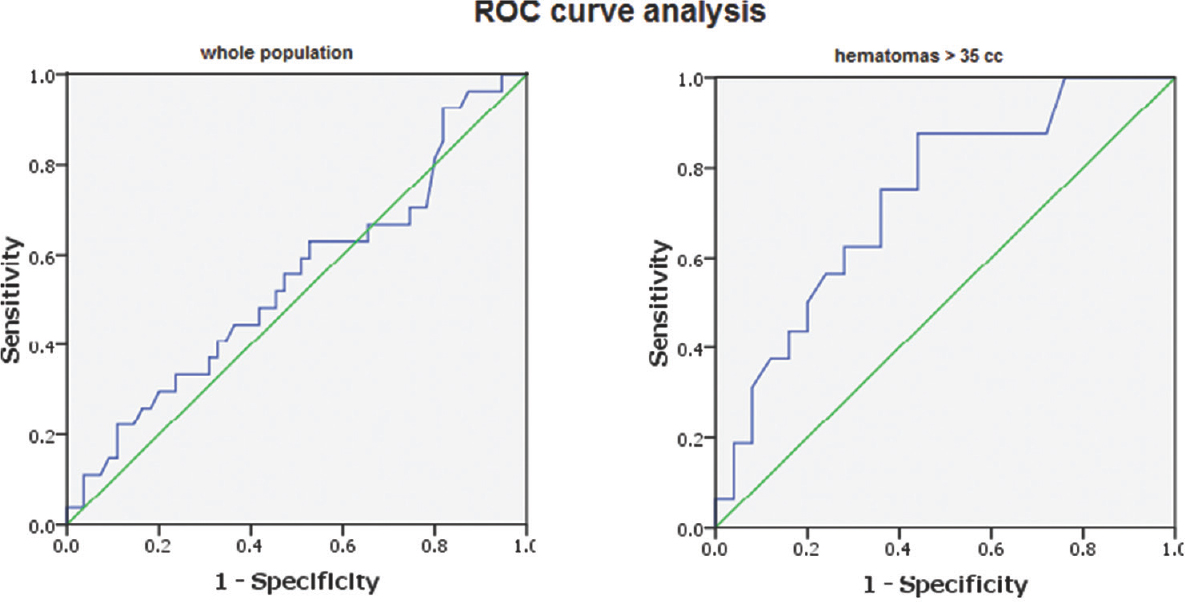
- Results of receiver operating characteristic curve analysis of time to surgery in outcome groups; in the whole patient population “left” and in clots >35 cc “right.” The area under the receiver operating characteristic curve revealed that the time to surgery has a weak predictive power of outcome among the whole group (area under the receiver operating characteristic = 0.542) but with good predictive power (area under the receiver operating characteristic = 0.732) in clots >35 cc
Exploring variables to mortality
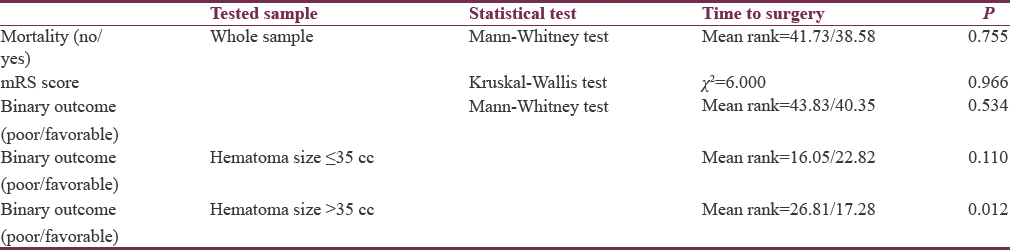
Six cases died during the follow-up period. The difference in time to surgery between dead and alive was not significant both among the whole population [Table 7] and within each size-split group (Mann–Whitney test, P = 0.133 and 0.120 for clots ≤35 cc and >35 cc, respectively). When ICH volumes and TVs of hemorrhages were compared among dead and alive, we found ICH volumes to have lower mean rank among dead cases; while TV to have higher rank among them, however, both differences were not statistically significant (Mann–Whitney test, P = 0.816, 0.202, respectively). On the other hand, rebleeding after surgical evacuation occurred in 4 (66.7%) out of the six cases of mortality, which was found to be of statistical significance (Fisher's exact test, P = 0.004).
DISCUSSION
Surgical evacuation of supratentorial hematomas still represents a controversial topic as surgical indications have not been finally defined.[252627] Our practice for managing supratentorial hematomas is to operate on a clot size >30 cc in a progressively deteriorating patient, except elderly patients in deep coma (GCS <8) with basal ganglionic hematomas. All cases in our institute, and so in this study, underwent standard craniotomy once surgical clot removal was indicated. The current guidelines suggest standard craniotomy only to be considered for patients with lobar hematomas >30 cc, within 10 mm of the surface.[2526] Other patient groups do not have recommendations for surgery, and other surgical methods including stereotactic aspiration and stereotactic endoscopic evacuation are not supported. Moreover, these guidelines did not recommend routine surgical evacuation of supratentorial ICH during the first 96 h.
The patient sample in our study represents a lobar hematoma subpopulation that might gain benefit from surgery[2526] and less likely to induce surgical uncertainty by neurosurgeons.[28] The sample included patients with lobar hematomas lying ≤1 cm from the cortical surface with clot sizes ≥30 cc. The size did not exceed 52 cc representing moderate-sized lobar hematomas. Exclusion of conscious patients who can be managed conservatively and comatose patients who are unlikely to profit from craniotomy made the finally included sample very likely to carry surgical benefits. We tried to explore the various causes of delayed surgical decision and its impact on outcome and mortality in this surgically promising category.
Surgical trial in intracerebral hemorrhage (STICH) and STICH II are international multicenter randomized trials that tried to answer the debates of surgical indication and surgical timing for supratentorial hematomas.[1529] In STICH trial, outcomes at 6 months revealed favorable outcomes in 122 (26%) patients allocated to early surgery (<24 h from randomization) versus 118 (24%) patients allocated to initial conservative treatment (odds ratio 0.89 [95% CI 0.66–1.19], P = 0.414). Moreover, the 6-month survival in the early surgery group did not significantly differ from initial conservative group. The final results revealed lack of sufficient evidence to justify early surgery compared with initial conservative treatment. A meta-analysis interpreting the results of STICH trial with respect to the previous nine randomized controlled trials including 2059 patients reported that although surgery was associated with a reduced risk of mortality and dependency, benefit from surgery was not robust and heterogeneity for mortality as an outcome was significant.[30]
However, the previously mentioned studies and others[31] suggested specific subgroups (such as lobar clots without IVH) that may benefit from early surgery strategy. These findings led STICH II trial to explore the benefit of early surgery in this subgroup of patients. However, they included a wide range of hematoma sizes (10–100 mL), as well as patients with wide range of GCS scores (conscious and unconscious). Although the study only reported and was based on the best eye (at least 2) and best motor (5 or 6) responses with no reference to the verbal response, using the same validated regression model as that used in our study to derive the verbal response[1718] predicts that the GCS scores in STICH II trial ranged from 10 to 15. In our study, the range of clot sizes was narrower allowing exploration of only moderate-sized lobar hematomas. Moreover, GCS scores ranged between 9 and 13, thus concentrating on specific category of consciousness which is thought to benefit the most from surgical intervention.[27] STICH II nonsurprisingly failed to find significant evidence to support early surgery versus initial conservative treatment in conscious patients with lobar hematomas between 10 and 100 ml and no evidence of IVH.[29]
Although comparison of time to surgery among outcome groups in medium-sized lobar hemorrhages in patients with presenting GCS scores 9–13 revealed no significant differences, comparison of such time window within clots ≤35 cc and those >35 cc revealed a difference. We found clots ≤35 cc operated early to carry a higher risk of rebleeding after surgery and rebleeding was higher among poor group. On the other hand, in clots >35 cc, poor prognosis was found to be associated with longer time to surgery when compared to favorable group. The cutoff time window associated with poorer outcome in this category was 11 h and 15 min. This may justify a recommendation to early interfere with hematomas >35 cc within the first 12 h of presentation to medical care, while safely conducting an initial conservative management in those ≤35 cc in size.
However, in this study, we did not exclude patients with intraventricular contamination as this was thought to unnecessarily reduce the final sample size. Instead, we just excluded cases with extensive IVH and those associated with hydrocephalus. According to the IVH score and formulas proposed and validated by Hallevi et al.,[19] the maximum IVH volume in our study was just 5cc and the maximum TV of hemorrhage was 57 cc. They reported that the TV of hemorrhage was correlated well to the outcome and mortality with a cutoff of 40 cc for poor outcome and 60 cc for mortality. This may explain the relatively lower mortality rate (7.3%) in this study compared to others (35%–52%)[2432333435363738] and why dead cases did not have significantly larger volumes of hematomas compared to survived cases. However, both ICH size and TV were found to be significantly higher among patients with poor outcomes, which agree with studies that reported ICH volume as a strong predictor of prognosis.[394041]
Serum glucose level was found to have no correlation to time to surgery indicating that it was of no significance in initial decision-making. However, a significantly bigger proportion of patients with poor compared to favorable outcome were detected among those with glucose levels higher than 180 mg/dL. This indicates that hyperglycemia is a significant indicator of poor outcome in ICH patients as also reported by others.[34424344] However, whether hyperglycemia directly contributes to less favorable outcome or exists as a consequence to stress response is not clear.
The main limitations of the present study included first being of a retrospective nature. Exploration of the selection bias that usually associated with retrospective studies was carried out by examining the association of various demographic, clinical, and radiologic variables to intervention groups which revealed no significant association, except for INR and associated chest problems. These two parameters were the most significant in delaying the decision of surgical intervention when abnormal. The second main limitation was missing of the accurate time of ictus as a referral point to time-to-surgery. The time of ictus was replaced by time of medical presentation as a reliable documented point of time for the study population that shared the same territory of residence with the same ambulance services.
CONCLUSION
Hemorrhage volume (ICH volume and TV), glucose level, and rebleeding after surgical evacuation were found to be significant predictors of poor outcome. The presence of minimal to mild (<1/3 of either lateral ventricle) IVH is not a predictor of mortality or poor outcome in medium-sized lobar hematomas; however, larger volumes are associated with poor outcome and should be considered in the plane of management. Early surgical evacuation of lobar hematomas >35 cc and initial conservative management for those ≤35 cc may be recommended for those unconscious patients with GCS scores of 9–13.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the administrative and information technology staff for maintaining the database and helping in data collection.
REFERENCES
- A prospective study of acute cerebrovascular disease in the community: The Oxfordshire Community Stroke Project–1981-86.2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53:16-22.
- [Google Scholar]
- Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394-9.
- [Google Scholar]
- Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987-93.
- [Google Scholar]
- Primary intracerebral haemorrhage: A controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet. 1961;2:221-6.
- [Google Scholar]
- A prospective randomised trial of surgical and conservative treatment of hypertensive intracranial haemorrhage. Acta Acad Med Shanghai. 1992;19:237-40.
- [Google Scholar]
- The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. J Neurosurg. 1989;70:755-8.
- [Google Scholar]
- Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Arch Neurol. 1990;47:1103-6.
- [Google Scholar]
- Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: A randomized study. J Neurosurg. 1989;70:530-5.
- [Google Scholar]
- Surgical Treatment for Intracerebral Hemorrhage (STICH): A single-center, randomized clinical trial. Neurology. 1998;51:1359-63.
- [Google Scholar]
- Early surgical treatment for supratentorial intracerebral hemorrhage: A randomized feasibility study. Stroke. 1999;30:1833-9.
- [Google Scholar]
- Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: A multicenter randomized controlled trial (SICHPA) Stroke. 2003;34:968-74.
- [Google Scholar]
- Stereotactic aspiration of deep intracerebral hematomas under computed tomographic control: A multicentric prospective randomised trial. Cerebrovasc Dis. 2003;16S:57.
- [Google Scholar]
- Surgery in intracerebral hemorrhage. The uncertainty continues. Stroke. 2000;31:2511-6.
- [Google Scholar]
- Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): A randomised trial. Lancet. 2005;365:387-97.
- [Google Scholar]
- Perihematomal edema and functional outcomes in intracerebral hemorrhage: Influence of hematoma volume and location. Stroke. 2015;46:3088-92.
- [Google Scholar]
- Appropriate use of the Glasgow coma scale in intubated patients: A linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma. 1996;41:514-22.
- [Google Scholar]
- The conundrum of the Glasgow coma scale in intubated patients: A linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma. 1998;44:839-44.
- [Google Scholar]
- The IVH score: A novel tool for estimating intraventricular hemorrhage volume: Clinical and research implications. Crit Care Med. 2009;37:969-74, e1.
- [Google Scholar]
- Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1-5.
- [Google Scholar]
- Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889-94.
- [Google Scholar]
- Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783-7.
- [Google Scholar]
- Could stroke trials be missing important treatment effects? Cerebrovasc Dis. 2002;13:73-5.
- [Google Scholar]
- Design and analysis of phase III trials with ordered outcome scales: The concept of the sliding dichotomy. J Neurotrauma. 2005;22:511-7.
- [Google Scholar]
- Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001-23.
- [Google Scholar]
- Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-29.
- [Google Scholar]
- Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012;43:1496-504.
- [Google Scholar]
- Spontaneous intracerebral haemorrhage: A surgical dilemma. Br J Neurosurg. 1999;13:389-94.
- [Google Scholar]
- Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet. 2013;382:397-408.
- [Google Scholar]
- Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database Syst Rev. 2008;4:CD000200.
- [Google Scholar]
- Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: Results from the STICH trial. Acta Neurochir Suppl. 2006;96:65-8.
- [Google Scholar]
- Spectrum of primary intracerebral haemorrhage in perth, Western Australia, 1989-90: Incidence and outcome. J Neurol Neurosurg Psychiatry. 1994;57:936-40.
- [Google Scholar]
- Primary intracerebral hemorrhage in the Oxford-shire community Stroke Project. Cerebrovasc Dis. 1995;5:26-34.
- [Google Scholar]
- Long term survival after primary intracerebral haemorrhage: A retrospective population based study. J Neurol Neurosurg Psychiatry. 2005;76:1534-8.
- [Google Scholar]
- Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40:3567-73.
- [Google Scholar]
- Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010;9:167-76.
- [Google Scholar]
- Mortality after hemorrhagic stroke: Data from general practice (The health improvement network) Neurology. 2013;81:559-65.
- [Google Scholar]
- The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891-7.
- [Google Scholar]
- Factors associated with in-hospital mortality following intracerebral hemorrhage: A three-year study in Tehran, Iran. BMC Neurol. 2004;4:9.
- [Google Scholar]
- Prediction of functional outcome in patients with primary intracerebral hemorrhage by clinical-computed tomographic correlations. J Res Med Sci. 2012;17:1056-62.
- [Google Scholar]
- Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke. 2004;35:1130-4.
- [Google Scholar]
- The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology. 2003;61:1351-6.
- [Google Scholar]
- The deleterious effect of admission hyperglycemia on survival and functional outcome in patients with intracerebral hemorrhage. Stroke. 2012;43:243-5.
- [Google Scholar]






