Translate this page into:
Occipital Falcine Anaplastic Hemangiopericytoma Mimicking Meningioma
Address for correspondence: Dr. Davendran Kanesen, Department of Neuroscience, School of Medical Sciences, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia. E-mail: davendrankanesen@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The rarity of hemangiopericytoma (HPC) and its controversial histological classification result in its frequent misdiagnosis and thus make the treatment quite challenging. It is often difficult to distinguish these tumors from meningiomas based on clinical features and radiological findings. This is a case report of a man, diagnosed clinically and radiologically as meningioma, which turned out to be anaplastic HPC on histological examination. A 30-year-old man presented with 3 months of progressively worsening of headache and blurring of vision. Clinical examination revealed the right homonymous hemianopia with reduced visual acuity and papilledema bilaterally. Magnetic resonance imaging revealed a multilobulated and heterogenous extraaxial lesion attached to the occipital falx. It measured 9.0 cm (AP) × 5.5 cm (W) × 5.8 cm (CC) and expands bilaterally with major bulk on the left. An occipital craniotomy followed by a subtotal tumor excision was only achieved due to profuse bleeding intraoperatively. Histopathology confirmed an anaplastic HPC (WHO Grade 3). The importance of differentiation between HPCs and meningiomas cannot be overemphasized. A preoperative correct diagnosis is difficult, but it is important that it should be made. Multilobulated (mushroom appearance), prominent internal signal voids, relatively narrow dural attachment, and lytic destruction without calcifications are useful findings to distinguish HPCs from meningiomas.
Keywords
Anaplastic hemangiopericytoma
dural tail
meningioma
occipital falcine
staghorn
INTRODUCTION
Intracranial hemangiopericytomas (HPCs) are rare central nervous system (CNS) tumors arising from Zimmermann pericytes or mesenchymal cells.[1] It is often difficult to distinguish these tumors from meningiomas based on clinical features and radiological findings. In fact, HPCs were initially regarded as a variant of meningioma. However, detailed research has indicated that they are more similar to the peripheral soft tissue tumors. The first documented case of primary intracranial HPC was reported by Begg and Garret in 1954.[2] Since then, case studies have revealed that HPCs are more aggressive than meningiomas and have high rates of recurrence and distant metastasis, with local recurrence rates approaching 91% and a 15-year risk of distant metastasis of 70% after surgery alone. Although HPCs were thought to originate from pericytes in the intracranial compartment, they were usually found as dural-based masses, with nondural-based intracranial HPCs being extremely rare. Here, we describe a 30-year-old man who had a large occipital falcine anaplastic HPC, which was initially managed as meningioma.
CASE REPORT
A 30-year-old gentleman with no known medical illness presented with 3 months history of progressively worsening headache associated with blurring of vision. Headache was throbbing in nature, predominantly over the occipital and nape which later on progressed to be generalized. His visual symptoms were mainly inability to focus and blurred vision with intermittent episodes of reduced visual field over the right half. Otherwise, there were no other significant complaints or symptoms.
He was alert with a full Glasgow Comatose Scale. Clinical examination revealed reduced visual acuity and established papilledema bilaterally. He also had right homonymous hemianopia on visual field examination. His other cranial nerve and motor-sensory examination were unremarkable. There were no other significant neurological findings besides the visual impairment bilaterally. Biochemical investigations were unremarkable.
Computed tomography (CT) and magnetic resonance imaging (MRI) studies [Figures 1 and 2] revealed an multilobulated and heterogenous extraaxial lesion in the occipital falcine region, predominantly toward the left occipital lobe. This lesion measured 9.0 cm (AP) × 5.5 cm (W) × 5.8 cm (CC) and appeared to have originated from the occipital falx cerebri, expanding bilaterally with the majority of the mass displacing the left occipital lobe. It addition, it was observed that the lesion showed minimal perilesional edema and was compressing surrounding brain parenchyma and bilateral occipital horn ventricles. The tumor had heterogenous enhancement after gadolinium injection.
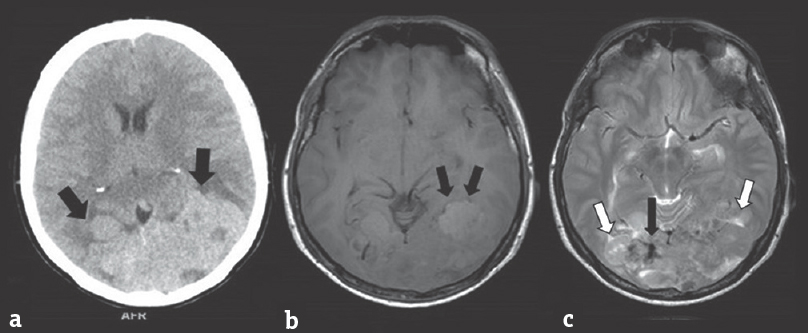
- (a) Noncontrasted computed tomography brain showing a multilobulated and isodense lesion over the occipital lobes bilaterally (black arrow). No tumoral calcification, osteolytic, or hyperosthotic bony change seen. (b) T1-weighted imaging on magnetic resonance imaging shows a well-defined irregular and lobulated mass, which appears heterogeneously isointense. The presence of “cortical buckling” supports the diagnosis of an extraaxial cerebral lesion (black arrow). (c) On T2-WI, a typical “sunburst” pattern is seen diverging radially into the tumor (black arrow). Also seen in this sequence are multiple cerebrospinal fluid clefts (white arrow) and areas of flow voids
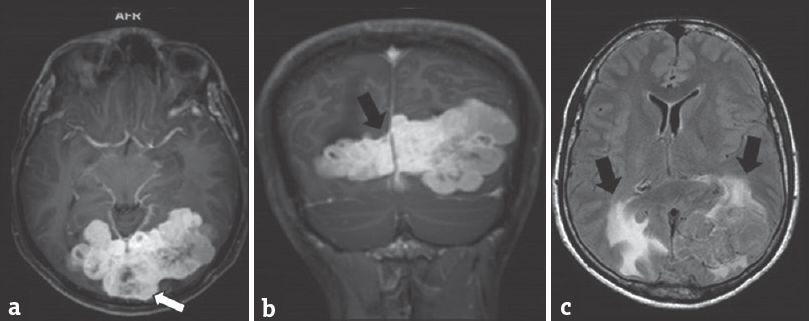
- (a and b) Contrasted magnetic resonance imaging studies shows vivid enhancement of the tumor. Scattered areas lacking enhancement are also seen (white arrow), probably due to necrosis, or hemorrhage within the tumor. Also seen here the “dural tail” sign and a narrow-based tumor (black arrow), at the falx cerebri where the tumor is attached. (c) On fluid attenuation inversion recovery sequence, the mass demonstrates some areas of hyperintensity in the periventricular and perilesional area suggestive of perilesional edema (black arrow)
A preoperative diagnosis of meningioma was considered. A standard occipital craniotomy was performed to assess the tumor. The tumor appeared to be well-encapsulated, gray in color, and highly vascularized. A subtotal tumor resection was done due to profuse bleeding intraoperatively. Postoperatively, he was monitored in the Intensive Care Unit and was eventually wean off ventilation and discharged home a week after his operation. He was discharged home well without any new focal deficit or worsening of the previous symptoms. The diagnosis of anaplastic HPC was confirmed through histopathological examinations [Figure 3], which showed a fairly circumscribed tumor composed of closely packed with moderate pleomorphic cells interlaced with blood vessels exhibiting staghorn pattern. These cells have round to oval nuclei, fine chromatin pattern, and prominent macronucleoli in areas. The cytoplasm is scanty, and the cells borders are indistinct. Occasional intranuclear cytoplasmic inclusion was also seen. No psammoma bodies were seen. Areas of tumor necrosis and hemorrhage are present. Mitotic figures were 5–7/10 high-power field. Staining with reticulin showed reticulin fibers wrapping the individual cells. Immunohistochemistry examination was positive for Vimentin and CD34, where else negative for epithelial membrane antigen, S-100, CD31, Desmin, CKA1 and A3, and glial fibrillary acidic protein. Ki-67 staining showed a proliferative index of 5%–8%. The findings were consistent with anaplastic HPC (WHO Grade III) which showed features of a high-grade lesion, such as mitosis >5/10 hpf, areas of necrosis, moderate nuclear atypia, increased cellularity, and areas of hemorrhage. He was subsequently referred to the oncology unit for further treatment and care.
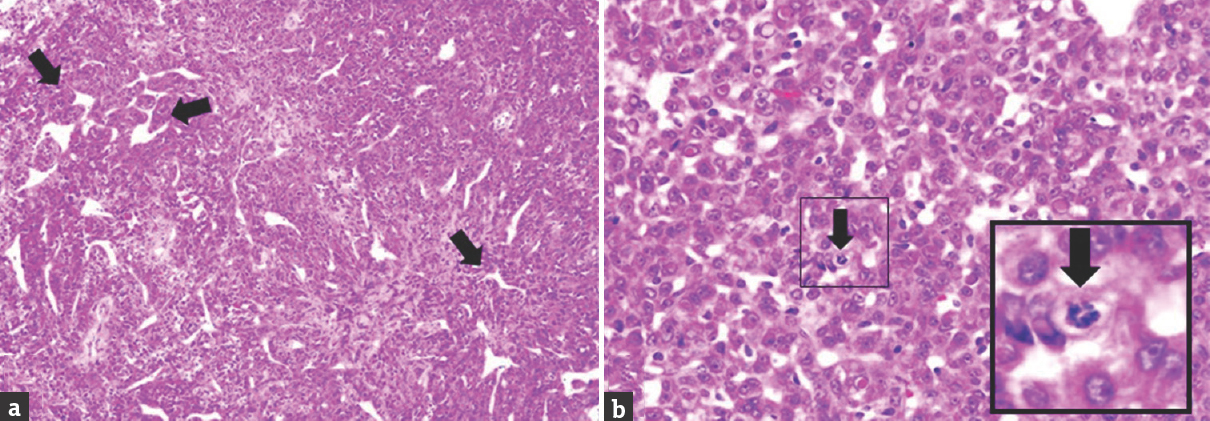
- (a) Histological section showing closely packed and moderately pleomorphic cells interlaced with blood vessels exhibiting staghorn pattern (black arrow) (H and E, ×10). (b) The cells have oval to round nuclei, fine chromatin pattern, and prominent nucleoli with moderate cytoplasm and indistinct cytoplasmic border. Occasional intranuclear cytoplasmic inclusion is seen. Mitosis is also seen here (black arrow) (H and E, ×40)
DISCUSSION
HPCs are rare intracranial tumors, with a reported incidence of <1%. Begg and Garret first reported the intracranial occurrence of HPC and documented its origin from the meninges. HPC's were earlier considered to be one of the variants of meningioma. Due to its distinct histomorphology, immunophenotype and biological behavior, WHO in 2007, laid down clear criteria for grading meningial HPC, making it a distinct entity.[2] Surgically, HPCs are described as well-demarcated masses attached to the dura and are associated with profuse bleeding on resection. These are aggressive lesions that tend to occur at an earlier age than other meningeal tumors, recur with high frequency, and metastasize extracranially, predominantly to bone, lung, liver, kidney, pancreas, and adrenals. Postoperative radiation therapy and/or chemotherapy have been associated with increased survival time, regardless of the histologic subtype of HPC.
Intracranial HPCs are detected at a mean age ranging from 37 to 44 years. In a pathologic series of 94 CNS HPCs, Mena et al. reported a mean age at presentation of 47 years among female subjects and of 41 years among male subjects. In contrast to meningiomas, which are more common in females, HPCs occur more often in males; the HPC male to female ratio is approaching 2:1. The average age of HPC onset is between 38 and 42 years, which is 10 years earlier than meningiomas.[3] Park et al. in 2013 following their series of 13 patients with CNS HPC reported a mean age of 48 years, with male predominance and majority were located at the parasagittal or falx.[4]
The location of intracranial HPCs is similar to that of meningiomas. Like meningiomas and other extraaxial masses, HPCs are dural-based and show white matter “buckling” as was seen in our case. New et al. described the three most common sites of 164 meningiomas as sphenoid/parasellar, lateral convexity, and superior parasagittal; no pathologic subtyping of meningioma was reported in this series.[5] Guthrie et al. reported that 27 of the 44 intracranial HPCs in their series were supratentorial; the four most common sites of occurrence were parasagittal/falx, convexity, posterior fossa, and tentorial; none occurred as purely intraparenchymal masses.[6] These findings and as seen in our case confirm that HPCs occur in locations similar to meningiomas.
Chiechi et al. reported, approximately one-third of their HPCs showed a narrow base of dural attachment, with the remaining two-thirds showing broad-based attachment. Chiechi et al. concluded in their series that HPCs may show a narrow base of dural attachment, a feature not typically seen in meningiomas.[7] This key feature was seen in our case following the MRI.
Reports of the CT appearance of intracranial HPC are scarce. HPCs, such as meningiomas, are extraaxial lesions, but unlike meningiomas, they do not have tumor calcifications. Servo et al. summarized their CT findings in eight cases of intracranial HPCs as follows: Unenhanced CT scans showed isodense or slightly hyperdense, well-defined, sometimes nodular masses, without calcifications, and these lesions were associated with slight edema. These HPCs were typically connected to the convexity or falx with a broad base, were often bilateral, and often showed dense, ring-shaped enhancement.[8] Retrospectively, these reports agree with some of our findings that intracranial HPCs are heterogeneous, hyperdense, dural-based lesions, and typically show heterogeneous enhancement on enhanced CT and MRI scans.
Descriptions of the MRI features of intracranial HPC are similarly limited. Cosentino et al. described a single giant intracranial HPC. The mass was heterogeneous, isointense with gray matter on T1-weighted images, slightly hyperintense on T2-weighted images, showed prominent internal vessel voids, and enhanced heterogeneously on contrast.[9] Retrospectively, our MRI findings concur with these reports.
In conclusion, intracranial HPCs are dural-based hypervascular masses similar to meningiomas; however, histologically, they are not meningiomas, and they often have different CT and MRI features. Intracranial HPCs are rare, extraaxial, multilobulated masses that typically occur in patients in their third and fourth decades. Although meningiomas are frequently associated with dural invasion and with the development of abnormal vessels, HPCs are more aggressive, tend to recur even after gross total resection, and occasionally have extracranial metastases. Unlike meningiomas, which frequently show hyperostosis of adjacent bone and may have intratumoral calcifications on CT scans, HPCs occasionally have bone erosion and lack calcifications and hyperostosis. Meningiomas typically show a broad base of dural attachment on CT and MR studies; therefore, if a dural-based mass has a narrow attachment, one should also consider the possibility of HPC. Unenhanced CT scans of HPCs typically show hyperdense heterogeneous tumors, and T1-weighted and T2-weighted MR images typically show heterogeneous isointense tumors. HPCs show heterogeneous enhancement on both contrast-enhanced CT scans and MR images.
Due to relatively high tendency of intraoperative bleeding of HPCs and recurrence even after gross total resection, the importance of differentiation between HPCs and meningiomas cannot be overemphasized. Preoperative correct diagnosis is difficult, but it is important that it should be made. Multilobulated (mushroom appearance), prominent internal signal voids, relatively narrow dural attachment, and lytic destruction without calcifications are useful findings to distinguish HPCs from meningiomas. Histopathology is the gold standard for definitive diagnosis, and it is crucial to differentiate and precisely grade these tumors. In our case, patient required a second stage excision 3 months later due to tumor recurrence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
- [Google Scholar]
- Hemangiopericytoma of the central nervous system: A review of 94 cases. Hum Pathol. 1991;22:84-91.
- [Google Scholar]
- Clinical analysis of intracranial hemangiopericytoma. J Korean Neurosurg Soc. 2013;54:309-16.
- [Google Scholar]
- National cancer institute study: Evaluation of computed tomography in the diagnosis of intracranial neoplasms. IV. Meningiomas. Radiology. 1980;136:665-75.
- [Google Scholar]
- Meningeal hemangiopericytoma: Histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25:514-22.
- [Google Scholar]
- Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol. 1996;17:1365-71.
- [Google Scholar]
- Diagnosis of intracranial haemangiopericytomas with angiography and CT scanning. Neuroradiology. 1985;27:38-43.
- [Google Scholar]
- Giant cranial hemangiopericytoma: MR and angiographic findings. AJNR Am J Neuroradiol. 1993;14:253-6.
- [Google Scholar]






