Translate this page into:
Normotensive state during acute phase of hypertensive intracerebral hemorrhage
*Corresponding author: Sucharita Anand, Department of Neurology, All India Institute of Medical Sciences (AIIMS) Jodhpur, Jodhpur, Rajasthan, India. sucharita.anand@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Anand S, Choudhury SS, Pradhan S, Mulmuley MS. Normotensive state during acute phase of hypertensive intracerebral hemorrhage. J Neurosci Rural Pract 2023;14:465-9.
Abstract
Objectives:
Hypertensive hemorrhage is a leading cause of intracerebral haemorrhage (ICH), although some of these patients may not present with high blood pressure (BP) at the time of ICH.
Materials and Methods:
This retrospective study included patients with history of hypertension presenting with ICH. Patients with systolic BP recording of more than 140 mmHg were included in hypertension group (group I). Patients whose BP rose to hypertension range after fluid correction were included in group II and patients with BP <140 mmHg on consecutive 1-week BP recordings were included in group III. Clinical features including volume of ICH of all the three groups were noted. Outcome in the form of mortality was analyzed. Chi-square test was used for categorical variables and independent t-test for continuous variables. P < 0.05 was considered significant.
Results:
Ninety-two ICH patients with history of hypertension were included in the study. Of them, 20 patients (22%) presented with BP <140 mmHg systolic at the time of ICH. After fluid correction, it rose to hypertensive range in 9 (10%) but remained normal in 11 patients (12%) during consecutive recordings for 1-week post-admission. On comparing normotensive and hypertensive groups, significant difference was seen in survival and volume of ICH.
Conclusion:
There is a subset of hypertensive patients who may present with normal BP recording during acute ICH. The BP rises subsequently with the correction of hypovolemia in some. The volume of hemorrhage in normotensives is relatively small but whether this translates into better prognosis needs further studies.
Keywords
Intracerebral hemorrhage
Blood pressure
Hypertension
Normotensive
INTRODUCTION
Intracerebral haemorrhage (ICH) is a leading cause of stroke constituting approximately 15% of total stroke.[1,2] It is a major cause of mortality, that is, only one-third of patients survives. Out of these, only 20% cases regain functions enough to lead an independent life. Rest of them remain incapacitated increasing morbidity further.[1] Majority of primary ICH are caused by hypertension followed by amyloid angiopathy.[3,4] However, at times, hypertension remains undiagnosed and presents for the first time as intracerebral hematoma. A subset of these patients have been assessed to have normal blood pressure (BP) at the time of presentation to the hospital[5] and if it is an undiagnosed case, diagnosis of hypertensive hemorrhage becomes difficult. Other important factors for ICH are age (34% in patients are above 80 years of age), male sex, and black population (compared to age-matched white population).[6-9] A recent epidemiology-based systemic review showed incidence of hypertensive ICH to be 51.8/100,000 person years in Asian population.[8] Majority of the ICH patients present with high BP recordings requiring lowering of BP to minimize hematoma volume expansion and reduction in mortality. However, some patients may present with normal BP at the time of ICH.[10] There may be fluctuation in BP, which itself is a poor prognostic marker. Therefore, we planned to study the acute ICH patients presenting with normal BP at the time of admission to the hospital and tried to delineate their clinical features. The BP was considered normal when systolic BP (SBP) was below 140 mmHg according to ATACH-II trial.[11]
MATERIALS AND METHODS
This retrospective study was carried out in neurology department of a tertiary care center of Northern India. The data of all the patients presenting with the primary ICH and known history of hypertension over a duration of 6 years (2014 to 2020) were collected. Their demographic profile, clinical features, consecutive 7 days’ post admission BP recording, Glasgow coma scale (GCS), modified Rankin Scale (mRS) at the time of admission and at the time of discharge, hematoma volume, and final outcome were noted. Patients of trauma or cerebral venous thrombosis, subarachnoid hemorrhage, and vasculitis were excluded from the study. Patients of acute stroke with computerized tomography or magnetic resonance imaging evidence of hemorrhage located at sites typical of hypertensive ICH were studied at eight-hourly basis for the pattern of BP for 1 week and for the need of anti-hypertensive agents during hospital stay. ABC/2 score was used for hematoma volume scoring.[12] SBP <140 mmHg was considered as normotensive BP.
Based on BP recording, three groups were made – group I had hypertensive range BP from the very beginning, group II had normotensive range of BP at admission with increase to hypertensive range within 3 days (during which no anti-hypertensive drug was used and fluid/electrolyte imbalance if any, was corrected), and group III had normal BP recording during 7 days of observation after admission.
The study was approved by the Institutional Ethical Committee and the IEC code was 2018-153-DM-EXP-3.
Analysis
The data were analyzed using SPSS version 23. Mean, median, and standard deviation were calculated. Clinical features between hypertensive and patients with normal BP were compared using chi-square test for categorical variables and independent t-test for continuous variables. P < 0.05 was considered significant.
RESULTS
In the study period mentioned, 92 patients (mean age 59.310.4, range 30–83 years, females 23 i.e., 28.3%) were included. Seventy-two patients were in group I, that is, hypertensive group. Twenty-two percentages (n = 20) patients had normal BP recording at the time of admission to the hospital. Out of them, nine patients (10%) had rise in BP to hypertensive range within 24–72 h of admission (group II). Remaining 11 patients (12%) were included in normotensive group, that is, group III. Anti-hypertensive drugs were started or increased at admission and thereafter in patients of hypertensive group (group I) and normotensive to hypertensive BP group (group II) whenever more than desired BP was noted. Demographic and clinical parameters among various groups are shown in [Table 1]. Pattern of mean BP recordings of these three groups is shown in [Figure 1].
| Parameters | Group I (%) | Group II (%) | Group III (%) |
|---|---|---|---|
| Mean age | 60.6±10.3 | 56.3±4.6 | 54.3±7.9 |
| Total number of patients | 72 | 9 | 11 |
| F/M | 21/51 | 0/9 | 2/9 |
| Diabetes mellitus as comorbidity | 9 | 0 | 5 |
| Number of patients who survived | 49 | 9 | 10 |
| Mean SBP | 179.8±22.9 | 127±10.9 | 125±7.1 |
| Mean DBP | 100.5±12.1 | 78.4±22.9 | 79±4.8 |
| Mean ICH | 24.7±12.3 | 19.6±5.5 | 16.7±5.8 |
| Location of bleed | Lobar 15 (16.3) | Lobar 3 (33.3) | Lobar 3 (27.2) |
| Putamen 17 (17.4) | Putamen 6 (66.6) | Putamen 4 (36.3) | |
| Thalamic 25 (27.2) | - | Thalamic 2 (18.2) | |
| Cerebellar 8 (11.1) | - | Cerebellar 1 (9.1) | |
| Primary IVH 3 (4.1) | - | - | |
| Brainstem 3 (4.1) | - | - | |
| Caudate 1 (1.4) | - | - | |
| mRS | |||
| 6 | 23 (31.9) | - | 1 (9.1) |
| 5 | 21 (29.2) | 2 (22.2) | 4 (36.4) |
| 4 | 22 (30.6) | 6 (66.7) | 6 (54.5) |
| 3 | 3 (4.2) | 1 (11.1) | - |
| 2 | 3 (4.2) | - | - |
| GCS | - | - | |
| 3 | 13 (18.1) | - | - |
| 4 | 2 (2.8) | - | 1 (9.1) |
| 5 | 6 (8.3) | 1 (11.1) | - |
| 6 | 7 (9.7) | - | - |
| 7 | 5 (6.9) | - | - |
| 8 | 11 (15.3) | 3 (33.3) | 2 |
| 9 | 5 (6.9) | - | 2 (18.2) |
| 10 | 2 (2.8) | 2 (22.2) | - |
| 11 | 5 (6.9) | - | 1 (9.1) |
| 12 | 7 (9.7) | 3 (33.3) | 4 (36.4) |
| 13 | 2 (2.8) | - | 1 (9.1) |
| 14 | 4 (5.6) | - | - |
| 15 | 3 (4.2) | - | - |
| Parameters | P-value on comparing Group I-III | P-value on comparing Group I-II | P-value on comparing Group I-II and III combined |
| Sex | 0.71 | 0.46 | 0.77 |
| Site of bleed | 0.43 | 0.38 | 0.43 |
| Age | 0.05 | 0.05 | 0.06 |
| mRS | 0.07 | 0.32 | 0.17 |
| GCS | 0.08 | 0.61 | 0.48 |
| Volume of ICH | 0.03 | 0.001 | 0.005 |
| No. of patients survived | 0.02 | 0.045 | 0.005 |
| Systolic BP | 0.001 | 0.001 | 0.001 |
SBP: Systolic blood pressure, DBP: Diastolic blood pressure, GCS: Glasgow coma scale, mRS: Modified Rankin Scale, BP: Blood pressure
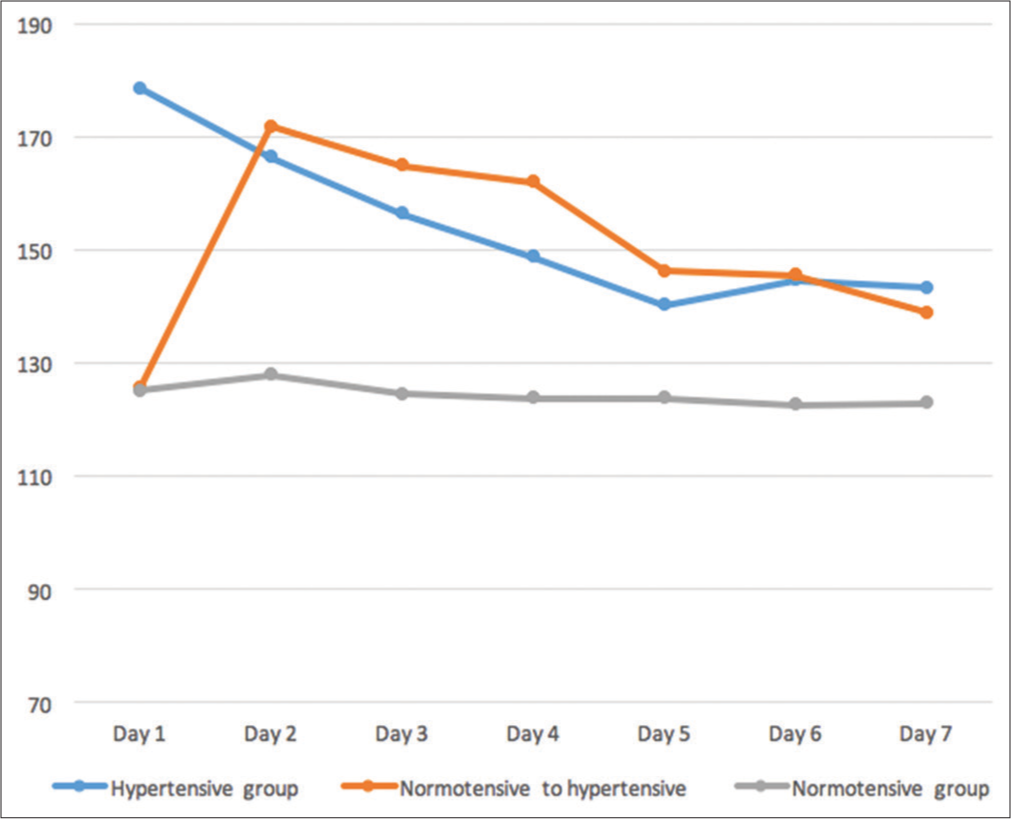
- Pattern of blood pressure in hypertensive (on antihypertensive drugs), normotensive to hypertensive (on antihypertensive drugs) and normotensive group (not on any anti-hypertensive drugs).
Comparison between ICH patients with hypertension and patients with normal BP group
Clinical characters were compared (1) between group I and II, (2) between group I and III, and (3) between group I and combined group II and III. Non-categorical variables were compared using Chi-square test. There was no significant difference seen in male and female sex and site of hemorrhage in between these groups. However, side of hemorrhage was more often left compared to right in group II and III while, in the hypertensive group I, it was nearly equally distributed between right and left side.
On comparing the survival between group I and group II, P value was 0.045 which was significant. Survival analysis between group I and III showed P = 0.027. As group II and group III had normal BP at admission, when these were combined and compared with group I, P-value was and 0.005 for volume of ICH and 0.015 for survival suggesting lesser hematoma volume and better survival in ICH patients with normal BP at the time of admission.
Categorical variables were compared using independent t-test. There was no significant difference in age, mRS, and GCS between group comparisons [Table 1].
DISCUSSION
The SBP plays an important role in management of the patients presenting with acute ICH. The rise in BP during acute ICH is attributed to raise intracranial pressure, premorbid hypertension, brainstem compression causing Cushing-Kocher reflex, release of neurotransmitters such as brain natriuretic peptide, and catechol-amines due to impaired sympathetic and parasympathetic pathways.[13-15] The effect of lowering the SBP helps in limiting hematoma volume expansion and edema formation in initial 24 h, thus reducing the mortality.[16,17] However, information is limited in patients presenting with normal SBP at the time of acute ICH. These patients may pose a challenge for the treating physician both in term of etiological diagnosis and management. Grose et al.[10] showed that 16% of the patients with acute ICH were normotensive. In our study, we found that 20 patients (22%) presented with SBP of equal or <140 mmHg. Out of these 20, 11 remained normotensive during 7 days of critical observation while the remaining nine developed hypertension during the observational period. The possible reason for normal BP recordings in these nine patients, who became hypertensive during observational period, could be the use of antihypertensive drugs that the patient might have been taking just before the ictus. Among the persistently normotensive 11 patients, five patients were the cases of diabetes mellitus, and thus, autonomic neuropathy may be a reason for absence of raised BP at the time of ICH. However, our patients did not show any hypertensive BP recording on consecutive monitoring despite not taking any anti-hypertensive medications. The other possible hypothesis is pressure effect on hypothalamus which caused autonomic failure and thus prevented reflective rise in BP during the time of acute ICH. A similar kind of prospective study showed that the percentage of patients presenting with normal BP was around 11% during acute ICH.[5] These patients showed BP recording in hypertensive range approximately after 3–5 weeks of ICH. It is possible that the return of BP to original hypertensive state had occurred following resolution of brain edema during the said period. However, retrospective nature of the study limits the follow-up of these patients.
Our study showed that group of patients with normotensive BP at the time of ICH had more survival compared to the other group. This was true even for patients who had normal BP at the time of ICH, but subsequent BP recordings were in hypertension range. The mechanism responsible for this could be that the lower SBP prevents the hematoma volume expansion. Although we do not have the data of mean hematoma volume expansion, the mean ICH in all the groups also showed significant difference (P = 0.001) between mean volume of ICH in these two groups. Thus, lower hematoma volume expansion and edema formation may have attributed to survival of these patients. Therefore, the presence of normal BP at the time of ICH may be a good prognostic marker. The patients had lobar and putamen bleed in majority of the cases which are in accordance to the previous study. Lobar bleed is most commonly caused by cerebral amyloid angiopathy[18] which was not ruled out in our patients. However, history of hypertension favors the likely etiology to be hypertensive bleed.
These patients also undergo unnecessary investigations for establishing cause of ICH if history of hypertension is not present.
The limitation of our study was retrospective data and small sample size. This finding requires further studies for establishing the guidelines for managing normotensive BP state during acute ICH.
CONCLUSION
There is a subset of hypertensive patients who may present with normal BP recording during acute ICH. The BP rises subsequently with the correction of hypovolemia in some. Irrespective of the subsequent rise in BP, the size of hematoma remains small and the survival better in those who present with normal BP at the time of hemorrhage. As we could not do functional outcome analysis in this retrospective study, further studies are needed to answer whether the smaller volume and better survival translates into better overall outcome or not.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Primary intracerebral haemorrhage in the oxfordshire community stroke project. Cerebrovasc Dis. 1995;5:26-34.
- [CrossRef] [Google Scholar]
- A prospective multicenter study to evaluate the feasibility and safety of aggressive antihypertensive treatment in patients with acute intracerebral hemorrhage. J Intensive Care Med. 2005;20:34-42.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the stroke council, American heart association. Stroke. 1999;30:905-15.
- [CrossRef] [PubMed] [Google Scholar]
- Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536-50.
- [CrossRef] [PubMed] [Google Scholar]
- Normotensive state during acute phase of hypertensive intracerebral haemorrhage. Abstracts of the XVIIIth world congress of neurology. J Neurol Sci. 2005;238(Suppl 1):S1-526.
- [Google Scholar]
- The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 1992;326:733-6.
- [CrossRef] [PubMed] [Google Scholar]
- Intracerebral hemorrhage In: Gorelick PB, Alter M, eds. Handbook of Neuroepidemiology. New York: Marcel Dekker. Inc.; 1994. p. :141-67.
- [Google Scholar]
- Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010;9:167-76.
- [CrossRef] [PubMed] [Google Scholar]
- Intracerebral hemorrhage in the very old: Future demographic trends of an aging population. Stroke. 2012;43:1126-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intracerebral hemorrhage patients presenting with normal blood pressure. J Hum Hypertens. 2016;30:352-3.
- [CrossRef] [PubMed] [Google Scholar]
- Blood pressure-attained analysis of ATACH 2 trial. Stroke. 2018;49:1412-8.
- [CrossRef] [PubMed] [Google Scholar]
- The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304-5.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome after medical reversal of transtentorial herniation in patients with supratentorial mass lesions. Crit Care Med. 2000;28:1556-64.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac effects of stroke. Curr Treat Options Cardiovasc Med. 2004;6:199-207.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma concentrations of brain natriuretic peptide in patients with acute ischemic stroke. Cerebrovasc Dis. 2005;19:157-64.
- [CrossRef] [PubMed] [Google Scholar]
- Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral haemorrhage. Stroke. 1998;29:1160-6.
- [CrossRef] [PubMed] [Google Scholar]
- Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636-41.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for lobar and non-lobar intracerebral hemorrhage in patients with vascular disease. PLoS One. 2015;10:e0142338.
- [CrossRef] [PubMed] [Google Scholar]






