Translate this page into:
Nonalcoholic Wernicke's Encephalopathy: A Retrospective Study from a Tertiary Care Center in Northern India
Address for correspondence: Dr. Irfan Ahmad Shah, G-19 Senior Resident Hostel, Sher-I-Kashmir Institute of Medical Sciences, Soura, Srinagar - 190 006, Jammu and Kashmir, India. E-mail: irfanskims@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The objective of this study was to describe the demographic features, clinical presentation, and management and outcome of fifty cases of nonalcoholic Wernicke's encephalopathy from a tertiary care hospital of a region with reported incidence of thiamine deficiency disorders.
Materials and Methods:
In a retrospective study, fifty adult cases of Wernicke's encephalopathy were analyzed. The diagnosis of Wernicke's encephalopathy was made according to the European federation of neurological societies guidelines 2010. Response to thiamine replacement and associated brain magnetic resonance imaging (MRI) findings were also considered as supportive evidence.
Results:
The mean age of patients was 50.38 years with 20 males and 30 females. The most common clinical manifestations were alteration in sensorium in 30 (60%), ataxia in 18 (36%), memory impairment in 15 (30%), nystagmus in 35 (70%), ophthalmoparesis in 11 (22%), and seizures in 4 (8%). A total of 42 patients had a history of recurrent vomiting. All patients had polished rice as their staple diet. Thirty-five patients had associated polyneuropathy and 15 had a gastrointestinal disorder. Twenty patients underwent MRI which showed both typical and atypical lesions. Majority of patients showed partial or complete response to intravenous thiamine. On discharge, the most common residual symptoms were lower limb weakness, ataxia, and memory impairment.
Conclusion:
The study shows high incidence of nonalcoholic Wernicke's encephalopathy in the region with predominant causative factor being a thiamine deficient diet. Recurrent vomiting can be a prominent early symptom of thiamine deficiency and its recognition can help in the early diagnosis of Wernicke's encephalopathy and related thiamine deficiency disorders. Thiamine fortification of food should be done in areas with reported incidence of thiamine deficiency disorders.
Keywords
Beriberi
nonalcoholic Wernicke's encephalopathy
polished rice
thiamine deficiency
Wernicke's encephalopathy
INTRODUCTION
Wernicke's encephalopathy is an acute neurologic disorder with high morbidity and mortality. It is characterized by nystagmus and ophthalmoplegia, mental status changes, and unsteadiness of stance and gait.[1] The disorder results from a deficiency in Vitamin B1 (thiamine), which in its biologically active form, thiamine pyrophosphate, is an essential coenzyme in several biochemical pathways in the brain.[2] Thiamine represents an essential coenzyme in intermediate carbohydrate metabolism and is also an osmotic gradient regulator.[3] Its deficiency may cause swelling of the intracellular space and local disruption of the blood–brain barrier.[4] Thiamine deficiency leads to brain lesions in selective, vulnerable regions, with high thiamine turnover within 2–3 weeks.[5] This duration is related to the time required to deplete the body's thiamine stores, which last for only up to 18 days.[6]
Autopsy studies have revealed a higher prevalence of Wernicke's encephalopathy lesions (0.8%–2.8%) than predicted by clinical studies (0.04%–0.13%).[789] It indicates that the disorder is still greatly underdiagnosed even in developed countries. Wernicke's encephalopathy is frequently associated with chronic alcohol abuse, but many other conditions can cause the disease. The settings related to nonalcoholic Wernicke's encephalopathy include staple diet of polished rice, disorders of recurrent vomiting or chronic diarrhea, chronic febrile diseases, renal diseases, cancer and chemotherapeutic treatments, gastrointestinal surgical procedures, magnesium deficiency states, and unbalanced nutrition.[10]
MATERIALS AND METHODS
The study was a retrospective analysis of fifty nonalcoholic patients admitted with a diagnosis of Wernicke's encephalopathy in the Neurology Department of a tertiary care center of Kashmir valley of Northern India, over a period of 4 years and 6 months from June 2011 to December 2015. The data were reviewed for demographic features, clinical history, physical signs, associated medical conditions, imaging findings, treatment, and sequelae. The clinical diagnosis of Wernicke's encephalopathy was made according to the criteria of Caine et al. recommended by European federation of neurological societies (EFNS) guidelines 2010, which requires two of the following four signs; (i) dietary deficiencies, (ii) eye signs, (iii) cerebellar dysfunction, and (iv) either an altered mental state or mild memory impairment.[1112] Furthermore, response to thiamine replacement and associated brain magnetic resonance imaging (MRI) findings were also considered as supportive evidence for diagnosis. Patients having suspected thiamine-related neuropathy with nystagmus only were not included in the study. MRI was performed during the acute phase of the disease at field strength of 1.5 T in twenty patients. All patients received intravenous thiamine initially for 5–10 days followed by oral maintenance.
RESULTS
The mean age of patients was 50.38 years (range 23–80 years). There were almost an equal number of patients in the age groups of 30–39, 40–49, 50–59, and 60–69 years. Males were twenty and females were thirty. Two females were pregnant and two were in postpartum state. The mean duration of hospital stay was 8.9 days (range 05–20 days). All the patients were nonalcoholic.
Alteration in sensorium was the presenting symptom in 30 patients; among these, 21 patients had acute confusion state, 7 patients were stuporous, and 2 were in coma. Memory loss was the presenting feature in 15 patients, all of whom had impairment in remembering recent events. Six patients had a history of diplopia. History of seizures was present in 4 patients and all had generalized tonic–clonic seizures. Nystagmus was the most common sign in our patients. Bilateral lateral rectus palsy was the most common form of ophthalmoparesis in 6 patients and 1 patient had internuclear ophthalmoplegia. The triad of Wernicke's encephalopathy was seen in only 5 patients. Two patients had concomitant congestive heart failure. Thirty-five patients had a history of lower limb weakness at presentation, among which 30 patients underwent nerve conduction studies which showed features of axonal polyneuropathy in all of them. One patient was admitted twice in a year with Wernicke's encephalopathy [Table 1].
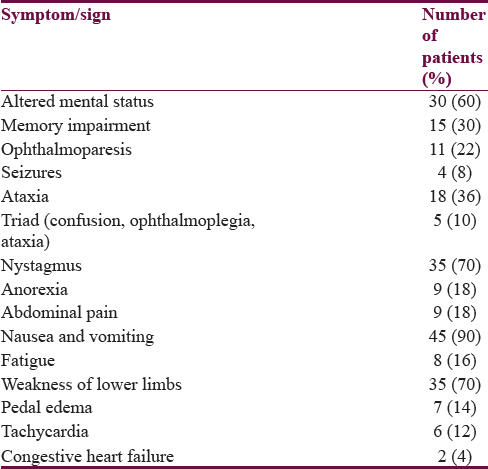
A history of preceding or concomitant recurrent vomiting was present in 42 patients. Three patients had nausea without vomiting. The average duration of vomiting was 27.2 days (range 2–90 days). Nine patients had vomiting of more than 45 days. However, 5 patients had vomiting of 3 days or less. A history of anorexia and poor oral intake was found in 9 patients.
Historically, only a few patients had an apparent clinical condition responsible for the development of vomiting or Wernicke's encephalopathy. Two patients were pregnant among which one had hyperemesis gravidarum. Two patients were in peurperium. One patient had chronic diarrhea and two had a history of recent major surgery. Two patients had undergone gastrojejunostomy more than 5 years before presentation. One patient had received intravenous dextrose a day before presentation. All patients had polished rice as their main staple diet which is washed before cooking. Besides, the patients also gave a history of regularly consuming green tea and bread made of highly polished floor.
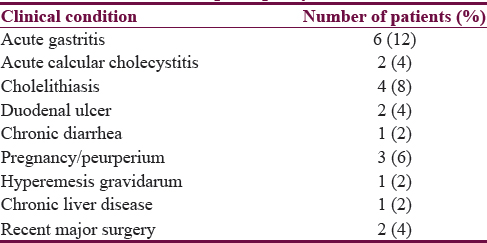
Based on the symptom severity, 33 patients were evaluated for vomiting and other gastrointestinal symptoms. Abdominal ultrasound was done in 23 patients; 16 had normal ultrasonography while in 7 patients, it was abnormal among which 3 had asymptomatic cholelithiasis. Upper gastrointestinal endoscopy was done in 20 patients, out of whom 12 had normal study while 8 had endoscopic lesions among which two had partially healed duodenal ulcer. Serum amylase was done in 10 patients, all had normal levels [Table 2].
On baseline evaluation, 8 patients had laboratory documented anemia with hemoglobin level ≤10 mg/dl and 6 patients had serum albumin level ≤3 mg/dl. Serum lactate levels at presentation was done in 12 patients, 4 had elevated levels. Serum thiamine levels were not checked in any patient as the facility was not available. Brain imaging was done in 34 patients; 13 underwent an MRI only; computed tomography (CT) scan only was done in 14 while both MRI and CT scan were done in 7 patients. CT scan was normal in 18 patients, 2 patients had bilateral thalamic hyperdensities, and 1 had subcortical hyperdensities, all were confirmed on MRI. Out of the 20 patients who underwent an MRI, 4 had normal MRI, 7 had single-site lesions, and rest had multiple lesions on T2 and/or fluid attenuation inversion recovery-weighted images. The most common lesions were bilateral medial thalamic hyperintensity in 11 patients followed by periaqueductal hyperintensities in 9 patients and tectal hyperintensities in 3 patients. Two patients each had lesions in cerebellum, basal ganglia, and subcortical white matter. Four patients with single lesions had an involvement of medial thalamic region, 2 had lesions in periaqueductal gray while 1 had in cerebellum. Three patients had repeat imaging after thiamine replacement, 2 had clearance of lesions, and 1 had partial resolution. Patients with alteration in sensorium at presentation had higher MRI positivity and more extensive lesions on MRI.
The dose of thiamine used for treatment varied from 300 mg to 600 mg/day initially given intravenously for 5–10 days followed by oral maintenance of 100–300 mg/day. Treatment was given as intravenous infusion 2–3 times a day. The most commonly used dosage regimen was 200 mg intravenously twice daily. Out of the 50 patients, 49 patients showed partial or complete improvement after treatment. One patient did not show any response and went on to develop Korsakoff psychosis. After an average hospital stay of 8.9 days, 9 patients had residual symptoms. The most common residual symptoms were lower limb weakness in 4, ataxia in 2, memory impairment in 2, and psychosis in 1 patient.
DISCUSSION
Our study presents one of the largest reported series of clinically diagnosed nonalcoholic Wernicke's encephalopathy in the world. The results of the study indicate that the disorder is a significant health problem in this community. Majority of the patients did not have any major disease known to be associated with Wernicke's encephalopathy such as cancer, gastrointestinal surgery, renal or psychiatric illness, or prolonged starvation.[10] Besides, the cohort did not have other features of malnutrition. This indicates that it is the diet that is selectively deficient in thiamine which is mainly responsible for this disorder in our studied patients. The population consumes polished rice as their main stable diet which is thiamine deficient. Home pounded rice was used in the past, but the practice has largely been abandoned. Furthermore, most people have a preference for bread made from polished wheat flour.[1314] People consume a good amount of green tea on daily basis which is reported to contain antithiamine factors and further contribute to thiamine deficient state.[15] Food faddism may also be contributory, especially in females and the elderly. Studies from the region have reported high incidence of infantile Wernicke's encephalopathy and thiamine responsive lactic acidosis in exclusively breast fed infants.[1617] This again highlights the state of thiamine deficiency of breast feeding mothers, which is a reflection of the nutritional status of general population. More females were affected by the disorder in our series which is in contrast to alcohol-related Wernicke's encephalopathy where higher incidence in males has been reported.[1] This may be due to poor nutritional status of the female population. Furthermore, pregnancy and breast feeding may contribute to the deficiency state in patients of reproductive age group.
Recurrent vomiting was the most common associated symptom in majority of the patients. Vomiting has been found to be both a cause and a manifestation of thiamine deficiency.[18192021] We believe that in our cohort, nausea and vomiting were mainly initial symptoms of thiamine deficiency as most of our patients did not have any identifiable cause for vomiting. Furthermore, the short duration and low frequency of symptoms in a substantial number of our patients suggest that these are only the manifestations of thiamine deficiency or its terminal precipitants as such duration is insufficient to deplete the body's thiamine stores.[6] The prominence of gastrointestinal symptoms in our patients may represent ethnic or genetic diffeferences in the initial manifestation of thiamine deficiency. Many of our patients historically resembled the reported cases of gastrointestinal beriberi where gastrointestinal symptoms are prominent.[222324] Gastrointestinal symptoms are also prominent in infantile Wernicke's encephalopathy which usually is related to consumption of thiamine deficient breast milk.[1925] We suggest that in thiamine deficient areas, any patient with persistent unexplained nausea and vomiting should be given a trial oral or intravenous thiamine.
Nystagmus was the most prominent sign. Nystagmus is likely an early sign of the effects of thiamine deficiency on central nervous system and should always be looked out in patients of unexplained encephalopathy as it favors the diagnosis of Wernicke's encephalopathy. A good number of patients present to our clinics with thiamine responsive neuropathy and nystagmus and likely represent a stage in the spectrum of progression of thiamine deficiency from peripheral to central nervous system. A high percentage of patients had associated peripheral neuropathy in our study. In one subset of the patients, the initial symptom was subacute onset weakness of lower limbs which progressed to ophthalmoparesis and encephalopathy. This implies that thiamine-related neuropathy and encephalopathy are a continuum of symptoms although many patients may manifest only with one of the two disorders.[26] Furthermore, there may be a delay in recognizing some of the vague symptoms of thiamine neuropathy, which lead to progression of the disease.[13]
Gastrointestinal disorders constituted the largest identifiable associated diseases in our study although no association was found in the majority of patients. These gastrointestinal disorders can precipitate an underlying thiamine deficiency state or if prolonged, they may by themselves induce thiamine deficiency.[1819] The supply of thiamine to the body can be reduced by chronic diarrhea or persistent vomiting. Hepatic disease reduces storage and interferes with thiamine metabolism. Patients with dyspepsia or gastritis may only take foods with better gastrointestinal tolerance and in the course avoid thiamine rich foods. Gastrointestinal disorders may also impair the absorption of thiamine.
On MRI, Wernicke's encephalopathy usually involves the medial thalamic region, periaqueductal gray matter, mammillary body, tectum and periventricular region, all of which are considered the typical sites of involvement.[27] Our patients had both typical and atypical findings of Wernicke's encephalopathy. The involvement of atypical sites such as caudate head, cerebellum, cortical, and subcortical is reported to be higher in nonalcoholic patients.[2829] Involvement of atypical sites is usually seen in association with typical sites and is thought to indicate progression of disease.[30]
Our patients showed a good response to treatment at a dose range of 300–600 mg of thiamine per day. The EFNS guidelines recommend that thiamine should be given 200 mg 3 times daily preferably through intravenous route and should be continued until there is no further improvement in signs and symptoms.[11] Similar regimen has also been proposed by Chataway and Hardman.[31] Considering our results, we advocate that a dose of 200 mg twice daily is sufficient for nonalcoholic patients. We advise maintenance dose of oral thiamine because the diet is thiamine deficient and there are chances of recurrence. Maintenance dose of oral thiamine may also be given to selected patients such as those with poor dietary habits or chronic dyspepsia. We cannot predict about the incidence of Korsakoff psychosis in our series as long-term follow-up was not available. Only one patient developed psychosis during hospital stay and all patients were adequately treated and showed good response; hence, the chances of developing Korsakoff psychosis were theoretically minimal.
Our study highlights thiamine deficiency as a major health problem in this part of the world. This can be attributed to many factors predominant being the diet. Since it is difficult to change the dietary habits of a population, we suggest that the major foods should be fortified with thiamine in this region. The mandatory enrichment of staple food with thiamine is operative in the United States, the United Kingdom, and parts of Europe.[32] In Australia, the enrichment of bread flour with thiamine has caused a 40% reduction of the incidence of Wernicke's encephalopathy and Korsakoff's syndrome.[3334] Furthermore, gastroenterologists and physicians should be made well aware of this condition as many patients present initially with gastrointestinal symptoms and timely intervention can help in preventing the development of Wernicke's encephalopathy. People at risk of developing thiamine deficiency should be given supplements of thiamine.
CONCLUSION
The study shows high incidence of nonalcoholic Wernicke's encephalopathy in the region with predominant causative factor being a thiamine deficient diet. Recurrent vomiting can be a prominent early symptom of thiamine deficiency and its recognition can help in the early diagnosis of Wernicke's encephalopathy and related thiamine deficiency disorders. The clinical presentations of Wernicke's encephalopathy can be varying and thus requires a high index of suspicion. Thiamine fortification of food should be done in areas with reported incidence of thiamine deficiency disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The Wernicke-Korsakoff syndrome. In: Vinken PJ, Bruyn GW, eds. Handbook of Clinical Neurology. Part II. Vol 28. Amsterdam: North-Holland Publishing Company; 1976. p. :243-70.
- [Google Scholar]
- Nutritional and metabolic disorders. In: Graham DI, Lantos PL, eds. Greenfield's Neuropathology Vol 1. (6th ed). London: Hodder Arnold; 1997. p. :601-52.
- [Google Scholar]
- Blood-brain-barrier disruption in acute Wernicke encephalopathy: MR findings. J Comput Assist Tomogr. 1991;15:1059-61.
- [Google Scholar]
- Hepatic and Wernicke's encephalopathies: Current concepts of pathogenesis. Am J Clin Nutr. 1980;33:2719-26.
- [Google Scholar]
- Thiamin. In: Shils ME, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health & Disease (9th ed). Baltimore, MD: Williams and Wilkins; 1999. p. :381-9.
- [Google Scholar]
- Clinical signs in the Wernicke-Korsakoff complex: A retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341-5.
- [Google Scholar]
- The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser. 1971;7:1-206.
- [Google Scholar]
- Early diagnosis of pediatric Wernicke's encephalopathy. Pediatr Neurol. 1999;20:289-94.
- [Google Scholar]
- Wernicke's encephalopathy: New clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442-55.
- [Google Scholar]
- EFNS. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408-18.
- [Google Scholar]
- Operational criteria for the classification of chronic alcoholics: Identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62:51-60.
- [Google Scholar]
- Thiamine Deficiency and Its Prevention and Control in Major Emergencies. WHO/NHD/99.13
- Beri-beri: The major cause of infant mortality in Karen refugees. Trans R Soc Trop Med Hyg. 2003;97:251-5.
- [Google Scholar]
- Antithiamins of plant origin: Their chemical nature and mode of action. Ann N Y Acad Sci. 1982;378:137-45.
- [Google Scholar]
- Thiamine responsive acute life threatening metabolic acidosis in exclusively breast-fed infants. Nutrition. 2016;32:213-6.
- [Google Scholar]
- Pyloric stenosis complicated by Wernicke-Korsakoff syndrome. Gastroenterol Hepatol. 1997;20:131-3.
- [Google Scholar]
- Wernicke encephalopathy – A severe neurological complication in a clinically inactive Crohn's disease. Eur Neurol. 2003;50:184-5.
- [Google Scholar]
- Outbreak of life-threatening thiamine deficiency in infants in Israel caused by a defective soy-based formula. Pediatrics. 2005;115:e233-8.
- [Google Scholar]
- Cerebral beriberi (Wernicke's encephalopathy); review of 52 cases in a Singapore prisoner-of-war hospital. Lancet. 1947;1:11-7.
- [Google Scholar]
- Gastrointestinal beriberi: A previously unrecognized syndrome. Ann Intern Med. 2004;141:898-9.
- [Google Scholar]
- Rare presentation of thiamine deficiency as gastrointestinal syndrome. Hawaii J Med Public Health. 2014;73:46.
- [Google Scholar]
- Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31:133-6.
- [Google Scholar]
- Superior hemorrhagic poliencephalitis (Wernicke's disease) occurring in an infant-probably due to thiamine deficiency from use of a soya bean product. Pediatrics. 1961;11:771-7.
- [Google Scholar]
- A cluster of polyneuropathy and Wernicke-Korsakoff syndrome in a bariatric unit. Obes Surg. 2002;12:328-34.
- [Google Scholar]
- Neuroimaging findings in acute Wernicke's encephalopathy: Review of the literature. AJR Am J Roentgenol. 2009;192:501-8.
- [Google Scholar]
- MR imaging findings in 56 patients with Wernicke encephalopathy: Nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009;30:171-6.
- [Google Scholar]
- Wernicke encephalopathy: Unusual findings in nonalcoholic patients. J Comput Assist Tomogr. 2003;27:235-40.
- [Google Scholar]
- Clinical characteristics and MR imaging features of nonalcoholic Wernicke encephalopathy. AJNR Am J Neuroradiol. 2008;29:164-9.
- [Google Scholar]
- Thiamine in Wernicke's syndrome – How much and how long? Postgrad Med J. 1995;71:249.
- [Google Scholar]
- The impact of fortified foods on total dietary consumption in Europe. Nutr Bull. 2004;29:188-98.
- [Google Scholar]
- Wernicke-Korsakoff syndrome in Sydney hospitals after 6 years of thiamin enrichment of bread. Public Health Nutr. 1998;1:117-22.
- [Google Scholar]
- Prevalence of Wernicke-Korsakoff syndrome in Australia: Has thiamine fortification made a difference? Med J Aust. 1998;168:542-5.
- [Google Scholar]






