Translate this page into:
Neurological manifestations of Graves’ disease: A case report and review of the literature
Address for correspondence: Dr. Swayamsidha Mangaraj, Department of Endocrinology, S.C.B Medical College, Cuttack - 753 007, Odisha, India. E-mail: drsmangaraj@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Graves’ disease (GD) is characterized by a hyperfunctioning thyroid gland due to stimulation of the thyroid-stimulating hormone receptor by autoantibodies directed against it. Apart from thyrotoxicosis, other clinical manifestations include ophthalmopathy, dermopathy, and rarely acropachy. GD is an organ-specific autoimmune disorder, and hence is associated with various other autoimmune disorders. Myasthenia gravis (MG) is one such disease, which is seen with patients of GD and vice versa. Though the association of GD and myasthenia is known, subtle manifestations of latter can be frequently missed in routine clinical practice. The coexistence of GD and ocular MG poses a significant diagnostic dilemma to treating physicians. The ocular manifestations of myasthenia can be easily missed in case of GD and falsely attributed to thyroid associated ophthalmopathy due to closely mimicking presentations of both. Hence, a high degree of the clinical vigil is necessary in such cases to appreciate their presence. We present a similar case which exemplifies the above said that the clinical challenge in diagnosing coexistent GD and ocular myasthenia.
Keywords
Graves’ disease
ocular myasthenia
ophthalmopathy
ptosis
Introduction
Graves’ disease (GD) is a common endocrine disorder and is the most common cause of spontaneous hyperthyroidism. It is associated with various autoimmune disorders such as myasthenia gravis (MG) and type 1 diabetes mellitus. The coexistence of ocular myasthenia in GD can present a diagnostic challenge as ocular symptoms in both diseases closely simulate each other.[1234] The diagnosis is essential from both therapeutic and prognostic point of view. An unrecognized and untreated hyperthyroidism aggravates myasthenia and can rarely precipitate a fatal myasthenic crisis. We report a case of a middle-aged female who presented with ocular symptoms and was subsequently diagnosed to have coexistent GD and MG.
Case Report
A 38-year-old female presented with drooping of both eyelids and double vision for 20 days. There was some improvement in her symptoms on immediately after getting up from sleep. On further inquiry, she gave a history of marked weight loss, tremulousness of hands, and the occasional palpitation for last 7 months. She had also noticed a swelling in the anterior neck for last 2 months. However, she had not sought medical help prior to it. There was no past history of thyroid or any autoimmune disorder in her and her first-degree relatives. She also had oligomenorrhea for last 9 months.
On examination, she was afebrile and had a thin built (body mass index - 17.8 kg/m2). She had a pulse rate of 120/min and normal blood pressure. There was a grade two diffuse nontender soft goiter palpable in the neck without any associated bruit. Respiratory and cardiovascular system examination revealed no abnormality. Neurological examination revealed the presence of bilateral symmetrical fine tremors of hands. She had bilateral ptosis with incomplete external ophthalmoplegia [Figure 1a]. Pupillary response and fundoscopy examination were normal. There was no evidence of any weakness in any other muscle group or any signs of bulbar muscle weakness. Biochemical investigations showed raised free triiodothyronine level of 11.86 pmol/L (normal: 3.10–6.80 pmol/L), raised free tetraiodothyronine level of 44.72 pmol/L (normal: 12–22 pmol/L), and a suppressed thyroid-stimulating hormone level of 0.039 mIU/mL (normal: 0.4–4.5 mIU/L). Hematological parameters, liver function tests, renal function test, and glycemic evaluation were normal. Ultrasonography of thyroid showed diffuse enlargement of the thyroid gland with increased intrathyroidal vascularity. The technetium (Tc-99m) thyroid scan showed diffuse increased tracer activity of the gland suggestive of hyper functioning of the gland. Fine-needle aspiration cytology of thyroid gland showed the presence of numerous follicles with scant colloid, classical fire-flare appearance, and lymphocytic infiltration. Magnetic resonance imaging study of the brain was normal, and that of orbit did not show any evidence of proptosis or extraocular muscle thickening [Figure 1b]. Repetitive nerve stimulation test (RNST) of bilateral facial nerve, right ulnar nerve, and left spinal accessory nerve showed no significant decrement after pre- and post-exercise stimulation. However, the patient showed significant improvement in ptosis and external ophthalmoplegia with neostigmine test [Figure 1c]. High resolution computerized tomography without contrast of thorax revealed the presence of a mildly enlarged thymus (antero-posterior diameter 18 mm) [Figure 1d]. Anti-acetylcholine receptor antibody (anti-AchR Ab) titre was also elevated 4.34 nmol/L (normal: <0.5 nmol/L). Based on the above findings a diagnosis of GD with ocular myasthenia was made. She was prescribed anti-thyroid drugs (tablet carbimazole) 10 mg thrice daily) and pyridostigmine (60 mg) thrice daily. On a 2 months follow-up, the patient showed considerable improvement in physical and biochemical parameters of thyrotoxicosis while mild ptosis and residual ophthalmoplegia still persisted.
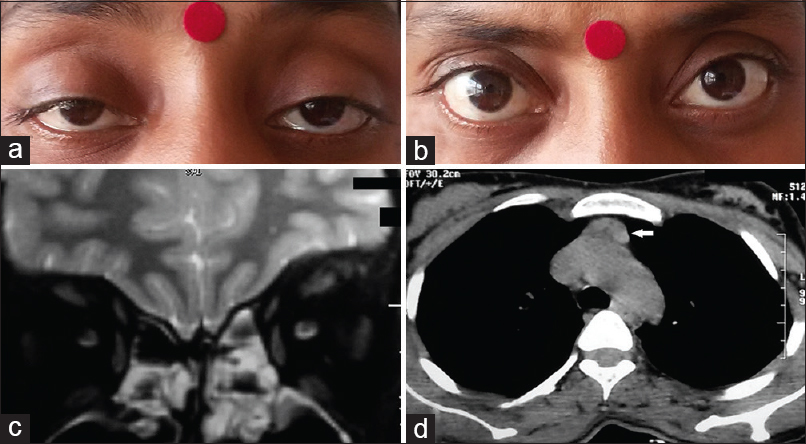
- (a) Clinical photograph showing the presence of bilateral ptosis. (b) Magnetic resonance imaging orbit is showing the absence of any extraocular muscle thickening. (c) Marked improvement in ptosis after neostigmine administration (positive response). (d) High-resolution computerized tomography thorax revealing mildly enlarged thymus gland (white arrow)
Discussion
GD is one of the common forms of autoimmune thyroid disease (AITD) and is the most common cause of thyrotoxicosis seen in clinical practice. Ophthalmopathy is one of the cardinals and striking features of GD and at times is the presenting feature of the underlying thyrotoxic state. GD is associated with various auto-immune disorders such as MG, type 1 diabetes mellitus, pernicious anemia, and autoimmune adrenal insufficiency. Epidemiological studies show that AITD occur in approximately 5–10% of patients with MG, whereas MG is reported in a fairly low frequency (0.2%) of patients with AITD.[4] AITD is more commonly associated with ocular myasthenia, and it is usually mild. The higher frequency of ocular MG in AITD could be that these disorders have a common genetic background.[5] In three-quarters of patients with both coexisting conditions, thyrotoxic symptoms occur before or concurrently with those of myasthenia.[6] Treatment of thyroid disorder in patients with MG leads to myasthenia regression in approximately two-third of the patients.[7] The ocular changes in GD may include exophthalmos, periorbital edema, lid lag, chemosis, and ophthalmoplegia. The extraocular muscles most commonly involved in GD are the superior and lateral recti.[7] The signs of ophthalmoplegia can occur due to infiltration of extraocular muscles as in GD or may be the sole manifestation of MG. Hence, these may pose a significant diagnostic difficulty to the treating physician. The presence of ptosis is a robust clinical clue to the possibility of myasthenia. Orbicularis oculi weakness in combination with ptosis or external ophthalmoparesis is a strong indicator of myasthenia.[89]
Repetitive nerve stimulation studies (RNS) and single-fiber electromyography (SFEMG) are recommended in the diagnosis of MG. The decremental response of muscle action potential amplitude in RNST is seen in only 33% of patients with purely ocular MG (OMG).[10] SFEMG has a sensitivity of 85–100% for OMG when used on the frontalis or orbicularis oculi muscle and a sensitivity of 91–100% in generalized MG.[11] The anti-AchR Abs have been demonstrated in as many as 80–99% of patients with generalized myasthenia and 30–77% of patients with OMG.[12] Fifty percent of patients presenting with ocular myasthenia develop generalized weakness within 6 months and up to 80% will generalize within 2 years.[13] Patient with ocular myasthenia without any progression for 2 years are likely to have symptoms restricted to the ocular muscles thereafter.[13] Hence, such patients require periodic follow-up to assess any progression. The treatment modalities of myasthenia include acetylcholinesterase inhibitors, corticosteroids, immunosuppressants, plasmapheresis, and thymectomy in selected cases.
Surgical orbital decompression is a well-accepted management strategy for GD with severe ophthalmopathy who are resistant to medical therapy or have a sight-threatening problem. Endoscopic method of orbital decompression provides an excellent visualization without external incisions and facilitates maximal decompression without the increased risk of hemorrhage, visual impairment, or infections.[14] In 1990, Kennedy et al. proposed to perform the Ogura technique transnasally under the endoscopic guidance.[15] The endoscopic approach allows surgeons to perform complete medial orbital wall decompression with excellent visualization of the key landmarks.[16] In a study by Baradaranfar and Dabirmoghaddam, the authors achieved an excellent results with an average retro displacement of 4.1 mm by endoscopic orbital decompression.[14] They also concluded that patients undergoing orbital decompression should be informed about the possibility of postoperative diplopia which usually resolves on its own.[14]
There is a high concentration of important structures in the narrow space of the orbital apex in the neighborhood of the optic canal, superior orbital fissure, and the infraorbital canal/groove (IOC/G) complex.[1718] The IOC/G complex is the pathway for the infraorbital bundle, and it is an important landmark in endoscopic transnasal decompression for the thyroid ophthalmopathy and other maxillofacial techniques.[16] In a recent study by Przygocka et al., the authors tried to evaluate the morphometric measurements of various anatomical orbital parameters. They found out that the mean distance from the inferior margin of the optic canal to: The posterior margin of the infraorbital groove measured at its medial border (designated as OC-S); to the posterior margin of the roof of IOC (designated as OC-C); and to the zygomaticoorbitale (designated as OC-ZO) on the right side were: 23.41 ± 3.10 mm; 34.44 ± 5.30 mm; and 47.53 ± 4.13 mm, respectively. Similar corresponding measurements (OC-S, OC-C, and OC-ZO) on the left side were 23.69 ± 2.80 mm; 36.75 ± 5.10 mm; 46.84 ± 3.24 mm, respectively.[16] Knowledge about these crucial anatomical measurements will serve as an invaluable guide for operating surgeon during dissection and will minimize major complications.
Conclusion
Hence, in spite of evaluating a case of GD with ophthalmopathy, the presence of ptosis should alert the clinician to explore the possibility of coexistent myasthenia especially ocular form. Furthermore, it should be borne in mind that myasthenia can precede or follow the diagnosis of GD in its clinical course.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ocular myasthenia gravis and Graves’ disease in a 10-year-old child. J Child Neurol. 2009;24:615-7.
- [Google Scholar]
- Ocular myasthenia gravis associated with euthyroid ophthalmopathy. Muscle Nerve. 2003;28:764-6.
- [Google Scholar]
- Concurrent presentation of ocular myasthenia and euthyroid Graves’ ophthalmopathy: A diagnostic challenge. J Clin Neurosci. 2008;15:719-20.
- [Google Scholar]
- Ocular myasthenia gravis coincident with thyroid ophthalmopathy. Neurol India. 2003;51:100-1.
- [Google Scholar]
- Mild clinical expression of myasthenia gravis associated with autoimmune thyroid diseases. J Clin Endocrinol Metab. 1997;82:438-43.
- [Google Scholar]
- The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477-588.
- [Google Scholar]
- Ocular myasthenia: Diagnostic and treatment recommendations and the evidence base. Curr Opin Neurol. 2008;21:8-15.
- [Google Scholar]
- Repetitive nerve stimulation in myasthenia gravis – Relative sensitivity of different muscles. Clin Neurophysiol. 2004;115:2776-82.
- [Google Scholar]
- Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Arch Neurol. 2003;60:243-8.
- [Google Scholar]
- Transnasal endoscopic orbital decompression in Graves’ ophthalmopathy. Arch Iran Med. 2004;7:149-53.
- [Google Scholar]
- Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg. 1990;116:275-82.
- [Google Scholar]
- Infraorbital groove localisation for the endoscopic decompression of the orbit in Graves’ disease. Folia Morphol (Warsz). 2015;74:78-83.
- [Google Scholar]
- Panoramic radiographic patterns of the infraorbital canal and anterior superior dental plexus. Dentomaxillofac Radiol. 1998;27:85-92.
- [Google Scholar]






