Translate this page into:
Multiple intracranial hemorrhages in pregnancy: A common autoimmune etiology
Address for correspondence: Dr. Hans Raj Pahadiya, Department of Medicine, Dr. S.N. Medical College Jodhpur - 342 001, Rajasthan, India. E-mail: drhans05sms@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disorder, primarily affect female in fertile age. Pregnancy in SLE female is a high-risk situation which can adversely affect maternal-fetal dyad. SLE can flare during pregnancy or in postpartum period. We describe a case of a young pregnant female who presented because of right hemiparesis due multiple hemorrhages in the brain. The first presentation of the SLE with multiple intracranial hemorrhages in pregnancy, preceding the other characteristic clinical symptoms is rare. Here, we high lighten the major neurological issues and maternal-fetal dyad issues in SLE pregnancy and treatment strategies for management of SLE in pregnancy.
Keywords
Fetal loss
intracranial hemorrhage
maternal-fetal dyad
pregnancy
systemic lupus erythematosus
thrombocytopenia
Introduction
Systemic lupus erythematosus (SLE) is a chromic, multisystem autoimmune disorder with a female preponderance, common in their teen to forties and diagnosed by the presence of standard criteria. Pregnancy in a woman suffering with SLE, have higher risk situation. Pregnancy can exacerbate or flare the SLE. The SLE adversely affects the outcome of the pregnancy. It can lead to maternal and fetal mortality and morbidity. The diagnosis of SLE in pregnancy is a tricky matter of identification and differentiation of disease flare from normal physiological changes of pregnancy. The neurological complication of SLE can be confused with the symptoms of eclampsia in pregnancy. The cerebrovascular accidents (CVAs) are common in the natural history of the SLE.[123] The infarctions are more common than hemorrhagic events, besides these, white matter changes, neuronal dysfunction, and psychological damage are underlying mechanisms for central nervous system manifestation of SLE.[4] The first presentation of SLE with intracranial hemorrhage (ICH) in the third trimester of pregnancy is a rare event. We explain the ICH in our case because of immune-mediated thrombocytopenia in a newly diagnosed case of SLE.
Case Report
A 35-year-old 9 months pregnant female, presented to our hospital because of history of weakness in the right half of the body with aphasia since 4 h. Her obstetric history was G3P2A0. There was no history of malar rashes, photosensitivity, joint pain, dryness of mouth, and gritty sensations in the eye or bleeding diathesis. She had no history of fetal loss in the earlier pregnancies, trauma in recent past and was not suffering from chronic illness. On examination, she was conscious, slightly confused, and understanding the commands but was not able to speak. There was no history of SLE and SLE pregnancy with CVA in her family. The vital parameters: Blood pressure was 122/82 mmHg, pulse rate 100/min, respiratory rate 18 breaths/min, and temperature was recorded 98°F by axilla. She had bilateral papilledema on fundoscopy. Other cranial nerve examination was normal. The meningeal signs were absent. There was hypotonia and power of 1/5 on all joints of the right half of body. Plantar reflexes were bilaterally extensor and deep tendon reflexes were decreased on the right side. Cardiovascular and respiratory system examination was not contributory. She delivered a full term baby through normal vaginal route, weighted 2.45 kg. Baby cried well at birth. The delivery was uneventful.
Hematology showed hemoglobin of 8.4 g/dL, leukocytes 17,960/mm3 and platelets of 62,000/mm3. The peripheral blood film examination showed normocytic normochromic red cells, normal differential count, and thrombocytopenia. The erythrocyte sedimentation rate (ESR) was 45 mm at 1st h. The blood sugar was 98 mg/dL, blood urea 50 mg/dL, creatinine 1.89 mg/dL, aspartate transaminase 50 IU/L, alanine transaminase 65 IU/L, serum lactate dehydrogenase 442 IU/L, total bilirubin 2.1 mg/dL, and total protein was 7.2 g/dL. The antinuclear antibody (ANA) level was 52 IU/ml (reference value 0–24 IU/ml), and anti-double-stranded deoxyribonucleic acid (anti-ds-DNA) was 172 IU/ml (reference value 0–25 IU/ml). Urinalysis showed proteinuria of 1+ and 24 h urinary protein was <0.5 g/dL. The chest X-ray, electrocardiogram, and echocardiogram did not reveal any significant abnormalities. The ultrasonography of abdomen showed mildly echogenic kidney with preserved corticomedullary differentiation. The magnetic resonance imaging of the brain had multiple confluent intraparenchymal T1/T2 hypointense lesions and peripheral fluid-attenuated inversion recovery hyperintensity, abnormal gradient susceptibility, and patchy areas of peripheral restricted water diffusion in the paramedian right frontal lobe (3.5 cm × 2.0 cm), left parietal lobe (2.5 cm × 2.5 cm), right temporal lobe (2.7 cm × 1.6 cm), and left cerebellar hemisphere (2.2 cm × 3 cm) suggestive of intraparechymal bleed. The MR venogram of brain vessels was normal [Figures 1 and 2]. Lupus anticoagulant, anticardiolipin, anti-Ro, and anti-La antibodies were negative. The coagulation profile including bleeding time, clotting time, prothrombin time, and activated partial thromboplastin time was within normal limit. The presence of anemia, thrombocytopenia, ANA and anti-ds DNA positivity, renal dysfunction and a vascular event fulfilled the diagnostic criteria of SLE. She was treated with mannitol, anticonvulsants, and steroid. At the follow-up of 2 months, she improved partially, and she could walk with support.
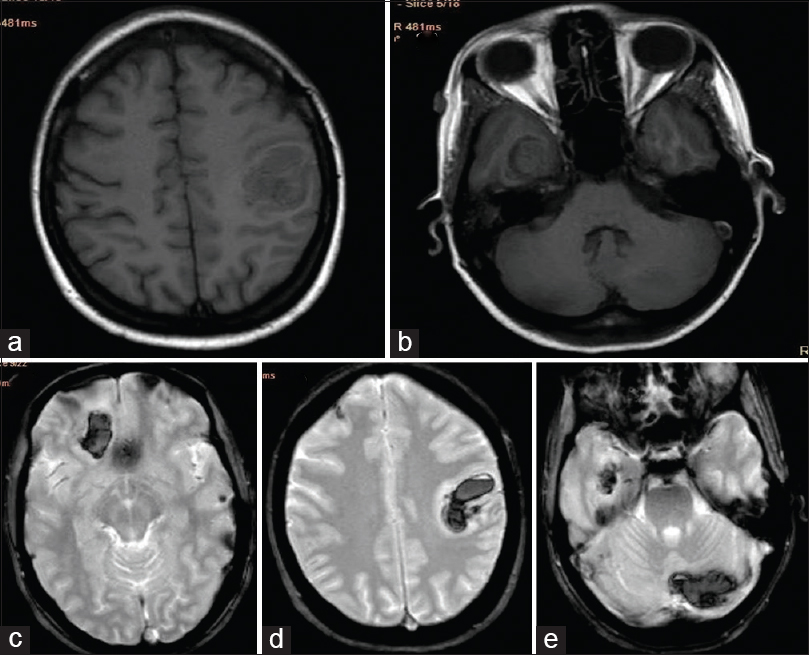
- Magnetic resonance imaging brain; (a and b) T1-weighted images-showing hypo-intense lesion in the left parietal and right temporal lobe; (c-e) T2 FFE images – showing hypo-intense lesion in the paramedian right frontal, left parietal, right temporal, and right cerebellar lobe
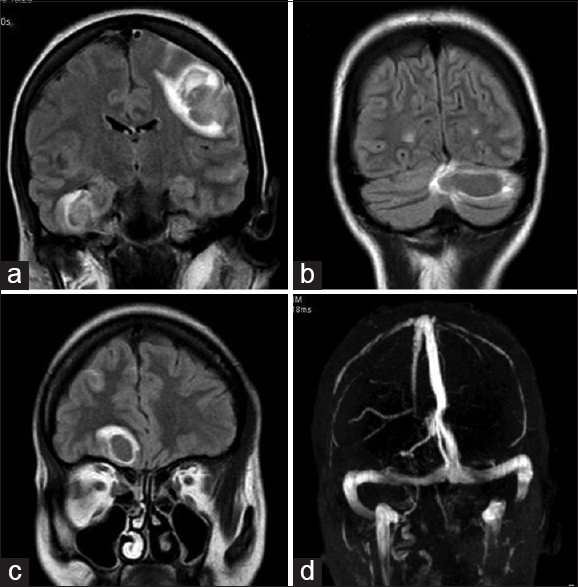
- Magnetic resonance imaging brain; (a-c) fluid-attenuated inversion-recovery images - showing fluid-attenuated inversion-recovery hyper-intensity, abnormal gradient susceptibility and patchy areas of peripheral restricted water diffusion in the paramedian right frontal lobe, left parietal lobe, left cerebellar hemisphere and right temporal lobe. (d) Magnetic resonance venogram of brain vessels was normal
Discussion
SLE is a chronic autoimmune multisystem disorder of predominantly childbearing age females. Among the connective tissue disorders SLE is frequently associated with neuropsychiatric complications. It can affect central as well as the peripheral nervous system. The pathogenesis of the cerebrovascular complications in SLE remains uncertain. The common neurological symptoms are cognitive dysfunction, including difficulties with memory and reasoning, headaches, seizures, psychosis, vasculitis, stroke, and myelopathy. The CVAs are common in the natural history of the SLE. A stroke occurs in approximately 5–20% of cases of SLE, of whom 0.4–7% have intracerebral hemorrhage and are associated with the mortality and morbidity of the disease.[34] The presence of hypertension, antiphospholipid antibodies, thrombocytopenia, vasculitis, anticoagulation therapy, aneurysmal rupture, or acquired deficiency of factor XIII contributes for the development of hemorrhagic diathesis in SLE.[567]
The SLE flare in pregnancy is still debatable. The risk of disease flare remains throughout the pregnancy and postpartum period. Lupus flare is common in the third trimester. The SLE flare usually present with multiorgan dysfunction in pregnancy. The common flare are cutaneous (rashes), musculoskeletal (arthralgia, arthritis), hematological (cytopenia), and renal (lupus nephritis). The possibility of flare is remained high in the patients who had active disease in previous 6 months before conception and elevated level of anti-ds DNA titer and low complement level.[8]
The diagnosis of lupus nephritis or active disease is difficult from the normal physiological changes of pregnancy. The lupus nephritis can be confused with symptoms of preeclampsia. The mild anemia, thrombocytopenia, hypertension, urinary albumin (<300 mg/dl), and normal to raised creatinine level are common in both the situations. The presence of elevated level of anti-ds DNA antibodies, low complement level, active urinary sediments, 24 h urinary calcium > 195 mg/dl, normal level of serum uric acid, and response to steroid favors to SLE. During pregnancy complement level can be falsely elevated because of estrogen-induced hepatic synthesis of complements.[128]
SLE is characterized by overproduction of autoantibodies because of loss of tolerance in both T cell and B cell compartment and resulted overproduction of B cells. Pregnancy is an immunocompromised state, and there are many immunological modifications and neuroendocrine changes in maternal environment to protect the fetus. Various cytokines and chemokines with T helper cells are key effectors of the immunological reaction. The pathophysiology of lupus flare is unknown. The various possible mechanisms are put forwarded:[8]
-
The Th1/Th2 cytokine shift is an important immunological modification during pregnancy. Th2 induce humoral immunity and antibody production whereas cellular immunity is stimulated by Th1. Th2 includes interleukin-4 (IL-4), IL-5, IL-6, and IL-10; and Th1 includes interferon-γ, IL-1, IL-2, IL-12, and tumor necrosis factor-α. Since SLE is mainly a Th2-mediated disease, during pregnancy a predominance of theTh2 response may be expected, making disease exacerbation more likely
-
The persistence low level of IL-6 in the third trimester of SLE pregnancy, as they increased in healthy controls, can lead to altered immune regulation from T cell to B cell. The IL-10 levels remained significantly elevated at conception, throughout pregnancy and postpartum in SLE patients as compared to normal pregnancy, resulting in continuous B cell stimulation. IL-10 act as a pleiotropic cytokine with both immune stimulatory and immune suppressive functions
-
Higher increase of Th17:IL-17 in SLE pregnancy can be associated with preeclampsia and pregnancy loss
-
Increased concentration of c4d, INF-α, ficolin-3 and increased various chemokines - CXCL8/IL-8, CXCL9/MIG, CXCL10/IP-10 in pregnancy are found to be associated with increased pregnancy complication and lupus flare
-
Lower levels of estrogens, progesterone, and impaired function of t regulator (Treg cells) in second and third trimester of SLE pregnancy are associated with impaired placental function and fetal loss
-
High prolactin level during pregnancy is also found to be associated with SLE flare in pregnancy. Prolactin can influence immune response and presence of anti-prolactin antibodies in few patients found to be associated with lesser chances of lupus symptoms.
After pregnancy, women with SLE have increased serum concentrations of CXCL8/IL-8, CXCL9/MIG, CXCL10/IP-10, and IL-10 chemokines compared with normal pregnancies, especially during active disease, hence flare may be in postpartum period also.
Pregnancy in a woman suffering with SLE, have higher risk situation. Majorly pregnancies terminate in normal live birth despite suffering with active disease or major organ involvement. The flare of SLE or active disease and lupus nephritis increase the risk of adverse outcome of pregnancy. The major fetal issues are higher risks of fetal loss, preterm birth, small for gestational age, intrauterine growth restriction (IUGR) and neonatal lupus syndromes. Mother can face with maternal death, preeclampsia, preterm labor, premature rupture of membranes, thrombosis, infection, and hematologic complications during SLE pregnancy. The presence of proteinuria, hypertension, thrombocytopenia, antiphospholipid antibodies, hypocomplementemia, and elevated anti-dsDNA antibodies level are other risk factor for fetal survival.[128]
The identification of cause for CVA in SLE pregnancy is a difficult task among various pathophysiological mechanisms of strokes - i.e., lupus flare, cortical vein thrombosis, eclampsia-preeclampsia, hypertension, or valvular heart disease related stroke.
The common hematological complications of SLE are-thrombocytopenia, leukopenia, lymphopenia, coombs positive hemolytic anemia, normocytic normochromic anemia, and increased ESR.[2] Thrombocytopenia is a risk factor for ICH. The presence of thrombocytopenia in pregnancy is multifactorial.[9]
Causes of thrombocytopenia in pregnancy:
-
Gestational (Incidental) thrombocytopenia
-
Other causes are:
-
Immune thrombocytopenic purpura
-
Immune-mediated - SLE and antiphospholipid antibody syndrome (APS)
-
Hemolysis - Microangiopathic hemolysis anemia, preeclampsia, Hemolysis Elevated Liver enzymes Low platelets syndrome, Hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, Disseminated Intravascular Coagulation, and Acute fatty liver of pregnancy
-
Viral-induced thrombocytopenia - HIV, hepatitis C virus, Epstein–Barr virus
-
Hypersplenism
-
Bone marrow dysfunction-aplastic anemia, megaloblastic anemia, hematological malignancies
-
Inherited type IIb von-willebrand disease and
-
Drug induced-heparin induced thrombocytopenia syndrome.
-
The individuals of SLE with thrombocytopenia bear a higher risk of end-organ damage throughout the natural history of disease which adversely affects the prognosis of disease. Thrombocytopenia is considered predominantly an immune-mediated process in SLE.[10] The peripheral destruction due to platelet autoantibodies, anti-thrombopoetin antibodies, lower effective circulating thrombopoietin, and impaired compensatory response due to bone marrow damage interact in SLE to ensue thrombocytopenia. These patients have more chances of CVAs, lupus nephritis, and other organ involvement.[11]
The management of SLE in pregnancy requires a multidisciplinary approach, with close monitoring and regular prenatal and postnatal follow-up for optimal outcomes. The treatment depends to the disease status and organ involvement, is limited to few selective safe drugs. If the patient has major organ involvement such as lupus nephritis, these patients should be continued on immunosuppressive medication to control the disease. The pregnancy should be planned in the state of controlled SLE status of at least 6 months duration before conception.[128]
The drugs including corticosteroids, azathioprine, hydroxychloroquine, cyclosporin, calcineurin inhibitor, sulfsalazine, and intravenous immunoglobulins (IVIg) may be used in pregnancy.
Corticosteroids are the choice of drug in active disease or flare. It decreases inflammation. The 90% dose of prednisolone is inactivated by placental hydroxylase (10% crosses placenta). Fluorinated steroid-dexamethasone, betamethasone crosses the placenta; hence, prednisolone in low doses (up 20 mg) should be given, and dexamethasone should be avoided. Short course of a high dose of methylprednisolone pulse therapy can be used in SLE flare. Fluorinated steroid can be used in a single dose in preterm delivery for the lung maturity. If fetus has complete heart block (CHB) then dexamethasone should be used. The adverse effects of steroid are maternal hypertension, gestational diabetes, eclampsia, preeclampsia, the premature rupture of membranes, fetal adrenal suppression, fetal immunosuppression, and neonatal cytomegalovirus infection. Prednisolone dose of >20 mg during late pregnancy can lead to IUGR/preterm.[12]
In the milder form of lupus pregnancy nonsteroidal anti-inflammatory drugs (NSAID) were used in the 1 and 2 trimester. In the last trimester, NSAID is associate with the premature closure of the patent ductus arteriosus, oligohydramnios, and fetal renal dysfunctions. The hydroxychloroquine should be used throughout the lupus pregnancy. The use of hydroxychloroquine is associated with reduced rate of lupus flare and decreased chances of fetal CHB and neonatal lupus syndrome during pregnancy.[1]
Azathioprine was considered safe; recently few report describe fetus IUGR, bone marrow suppression, and fetal immunosuppression. Hence, azathioprine may continue during pregnancy in a dose of <1.5–2 mg/kg/day, but one have to counsel with mother about the adverse effect of azathioprine. Sulfasalazine dose should not exceed 2 g. IVIg is more useful in lupus with APS, and in immune thrombocytopenia with hemorrhagic complication. Immunoglobulin G component of IVIg crosses the placenta, and reported to lead neuropsychiatric symptom in fetus in later life and fluid overload.[12]
The drugs cyclophosphamide, leflunomide, methotrexate and mycofenolate mofetil crosses the placenta and can lead to teratogenicity, fetal malformation, and fetal growth retardation. Hence, these drugs should be stop at least 3–6 months prior to the conception and contraindicated in the pregnancy. Among other medications - Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers, b-adrenergic blocker, statins, and warfarin are contraindicated, whereas Methyldopa, labetalol, nifedipine, and hydralazine can be safely used.
The thrombotic complication of lupus in pregnancy should be treated with low dose of aspirin ± heparin. Thrombotic event of APS should be treated with low-molecular-weight heparin throughout the pregnancy; anticoagulation should be discontinued just prior to delivery to avoid peripartum bleeding. Calcium supplementation should be routinely given to all SLE pregnant especially those receiving steroids and heparin.[12]
The maternal-fetal dyad should also be monitored carefully in postpartum period. SLE can flare in postpartum also. The drugs, i.e., cyclosporine A, cyclophosphamide, leflunomide, methotrexate, mycophenolate mofetil, and rituximab should be avoided. There should be at least 3 h interval between high dose of steroid and breastfeeding. In prenatal period mother should be kept in regular follow-up. Lupus pregnant females during the first trimester should be checked for complete blood count with differential counts and platelets, blood pressure, chest X-ray, renal function tests, liver function test, urine examination, antibody level (anti-ds-DNA, anti-Ro, anti-La, anticardiolipin, lupus anticoagulant, and protein S activity) and complement levels (C3, C4, CH50). The flaring of SLE and fetal complication depend on the antibody and complement levels during preconception period. The female with recurrent fetal loss should be evaluated for APS. The fetus of lupus pregnant female positive for anti-Ro and anti-La antibodies should be evaluated for cardiac defect with the help of fetal Two-dimensional echocardiography at 16 weeks of gestation thereafter every 1–2 weeks.[12]
Here, in our case, the presence of thrombocytopenia seems to be responsible for the development of the multiple hemorrhages. We considered thrombocytopenia because of the immune-mediated peripheral destruction of platelets, which was simultaneously diagnosed to having SLE. Our patient presented with neurological complications in the last trimester of normotensive pregnancy. Hence, patients presenting with hemorrhagic stroke having thrombocytopenia should be checked for an autoimmune disorder. The autoimmune diseases should also be kept as a differential diagnosis for thrombocytopenia in the pregnancy. Thrombocytopenia should be management carefully following the proper guidelines, which can decreases the thrombocytopenia related complications. In a case of lupus pregnancy, the maternal-fetal dyad should keep under proper antenatal checkups, prompt natal, and postnatal care with the use of selective safe drugs to achieve a good outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol. 2013;27:435-47.
- [Google Scholar]
- Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- [Google Scholar]
- The central nervous system in lupus erythematosus. Part 2. Pathogenetic mechanism of clinical syndrome: A clinical investigation. Rheumatology. 2002;41:619-30.
- [Google Scholar]
- Intracerebral hemorrhage in a patient with SLE and catastrophic antiphospholipid syndrome (CAPS): Report of a case. Clin Rheumatol. 2005;24:420-4.
- [Google Scholar]
- Intracranial hemorrhage in systemic lupus erythematosus associated with an autoantibody against actor XIII. Thromb Haemost. 2002;88:919-23.
- [Google Scholar]
- Understanding and managing pregnancy in patients with lupus. Autoimmune Dis. 2015;2015:943490.
- [Google Scholar]
- Immune thrombocytopenia in pregnancy. Hematol Oncol Clin North Am. 2009;23:1299-316.
- [Google Scholar]
- Thrombocytopaenia in lupus as a marker of adverse outcome – Seeking Ariadne's thread. Rheumatology (Oxford). 2006;45:1261-5.
- [Google Scholar]
- Suspects in the tale of lupus-associated thrombocytopenia. Clin Exp Immunol. 2006;145:71-80.
- [Google Scholar]






