Translate this page into:
Maintainence Treatment of Opioid Dependence with Tramadol
Address for correspondence: Dr. Siddharth Sarkar, Department of Psychiatry, National Drug Dependence Treatment Centre, Room No. 4096, Teaching Block, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029, India. E-mail: sidsarkar22@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Although tramadol has been used in the management of acute withdrawal in patients with opioid dependence, its use for maintenance treatment as a harm reduction approach has not been assessed systematically. This case series describes patients with opioid dependence who were treated with tramadol for long-term maintenance.
Methods:
Patients with opioid dependence who received treatment at the National Drug Dependence Treatment Centre of All India Institute of Medical Sciences, New Delhi, were included in the study. Patients who received at least 6 months of tramadol and had follow-up adherence of more than 80% were included in the case series.
Results:
A total of 25 cases were included, all of whom were males. The types of opioids being taken at the time of initiation of tramadol were natural opiates (poppy husk and raw opium), followed by heroin. The median dose of tramadol at initiation and maintenance was 300 mg/day. Nineteen patients were able to achieve complete abstinence to other opiates on tramadol.
Conclusion:
Tramadol may be an effective option in the long-term management of patients with opioid dependence. Further studies are required for establishing the efficacy of tramadol for agonist management of patients with opioid dependence.
Keywords
Harm reduction
opioid substitution
tramadol
INTRODUCTION
Opioid substitution therapy (OST) for treatment of opioid dependence has been developed a harm reduction measure with the aim of reducing the use of illicit opioids.[1] It has been found to be efficacious and cost-effective for the treatment of opioid dependence, with methadone and buprenorphine being well-demonstrated pharmacological options for OST.[234] However, even where effective maintenance treatments exist, many patients move between treatment modalities over the course of their treatment careers, depending on their needs and goals at the time.[5] Although methadone and buprenorphine are the two established options for OST,[56] concerns about diversion of these medications have been raised, leading to search for other options as well.[78]
Despite the scale up of OST services in India and South Asia, the overall coverage of OST remains low, and various barriers exist between service providers and recipients.[9] One of these barriers reported by the recipients is the dispensing pattern and having to come frequently to the centers for obtaining the mediation.[10] Hence, there is a need of exploring other options in the country for long-term management of opioid dependence. Tramadol is a partial opioid agonist that acts on the μ-receptor. The potency of tramadol has been reported to be considerably less as compared to morphine. Since tramadol has been shown to be an effective option for control of withdrawal symptoms,[111213] it could probably be used as an opioid agonist for long-term harm reduction approach as well. We present a case series from our center of the use of tramadol for maintenance long-term treatment for patients with opioid dependence.
METHODS
The present case series was conducted among the patients seeking treatment at the National Drug Dependence Treatment Centre (NDDTC), Ghaziabad, India. The NDDTC is a tertiary care treatment facility which caters to individuals with substance use disorders from North India. Patients can either seek treatment directly at the center or be referred from another treatment facility. The NDDTC offers both inpatient and outpatient treatment services and primarily caters to individuals with opioid and alcohol use disorders. The clientele of the center comprises primarily of patients from middle and lower socioeconomic status and the treatment is highly subsidized with medications being provided free-of-cost from the center.
For patients with opioid use disorders, the center offers both agonist and antagonist treatment. The agonist treatment comprises the option of buprenorphine primarily while the antagonist treatment comprises naltrexone. Due to concern of diversion of buprenorphine, the patients are usually dispensed daily for the initial couple of months, before being given as a “take home” medication. However, some of the patients with opioid dependence who express preference for agonist maintenance are not able to come for daily dispensing of buprenorphine. For such patients, limited doses of tramadol are given as an option as a take home medication, so that patients experience relief in their withdrawal symptoms. While some of the patients are able to taper off the tramadol prescribed over time, few are not able to reduce and taper off the doses of tramadol and continue to take tramadol, without needing to take recourse to illicit opiates. Such patients continue to receive tramadol for extended periods, with reduction or cessation of other opiate use, thus falling under the ambit of “harm reduction” approach.
The present case series reports patients with opioid dependence who had received tramadol at least for 6 months duration. The cases were identified by two of the investigators (VP and MV). Cases were included if they had been under treatment with tramadol for more than 80% of the duration of treatment at the center. Data regarding demographic details, duration of substance use, and the types of substances being taken were recorded. Data were also gathered regarding the reason of choice of tramadol as treatment agent, doses used, and outcome on this agent. The data were entered into an Excel chart and descriptive statistics was carried out.
RESULTS
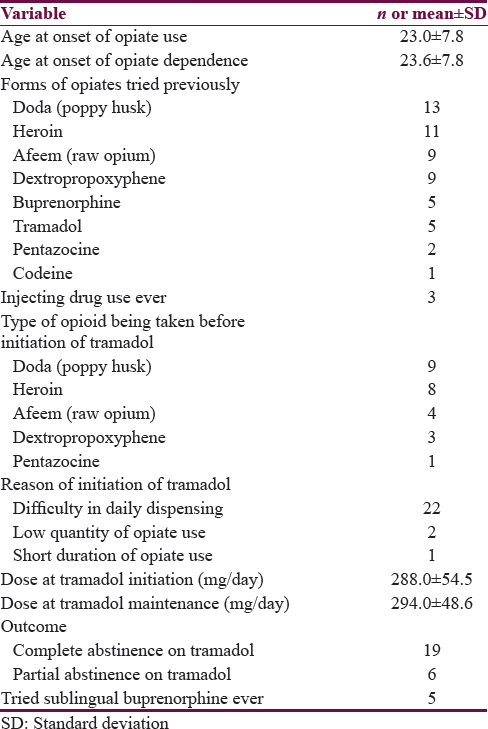
The demographic details and clinical characteristics of the 25 cases included in the series are depicted in Table 1. The mean age of the sample was 40.9 years, and all the cases were males. The sample was largely employed and educated up to 10th grade. All the individuals in the case series fulfilled the diagnosis of opioid dependence. The use of tobacco, alcohol, and benzodiazepines was more common than dependence on these substances, respectively.

The characteristics of the use of opiates are shown in Table 2. The largest proportion of patients was using natural opiates (poppy husk or raw opium) before initiation of tramadol, followed by heroin and then prescription opiates. The primary reason of starting tramadol was recorded to be difficulty in daily dispensing. The mean duration of receiving tramadol was 9.8 (±4.0) months (median 8 months, range 7–24 months). The median dose of tramadol initiation and maintenance was 300 mg/day (with a mean of 288 mg/day and 294 mg/day, respectively). While 19 patients attained complete abstinence (>1 month) to other opiates at least once, six attained partial abstinence (i.e., reduction of dose of other opiates or abstinence < 1 month). Even after achieving complete abstinence, 9 patients had relapsed to previous substance of use, with craving (n = 4), inability to follow-up (n = 2), withdrawal (n = 2), and peer pressure (n = 1). Five of the patients also had tried buprenorphine and four of them preferred buprenorphine over tramadol.
DISCUSSION
Although tramadol has been previously used for the management of acute withdrawals in patients with opioid dependence, this case series presents its effective use for long-term agonist management of patients with opioid dependence. The lower abuse potential of tramadol in humans might be beneficial during long-term management and can address an important barrier in OST implementation, i.e., “diversion” of dispensed medication.[1013]
A larger proportion of the patients who were maintained on tramadol in this study were those who were using natural opiates. As compared to heroin, patients with natural opiate use are probably able to maintain themselves on a less potent opioid agonist. However, this needs confirmation on a prospective comparative sample of heroin and natural opiate users who are prescribed tramadol. The doses currently used in this sample (i.e., a median of 300 mg/day) are probably safe as these doses are not associated with cognitive and psychomotor effects,[14] and seizures are likely to occur at higher doses.[15]
For a pharmacological agent to qualify for agonist maintenance for opioids, it needs to fulfill requirements such as reduction of craving and having limited salience for the patient (apart from pharmacological stability, agonist activity, and safety).[1] Tramadol was able to achieve complete abstinence for a considerable subset of the patients, and hence offers some promise as an agonist. Further research may yield comparative data of efficacy and safety of tramadol as an agonist for patients with opioid dependence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Response to commentaries on ’which medications are suitable for agonist drug maintenance’. Addiction. 2016;111:781-2.
- [Google Scholar]
- The effectiveness of opioid substitution treatments for patients with opioid dependence: A systematic review and multiple treatment comparison protocol. Syst Rev. 2014;3:105.
- [Google Scholar]
- Opioid substitution therapy in resource-poor settings. Bull World Health Organ. 2011;89:243.
- [Google Scholar]
- Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103:1484-92.
- [Google Scholar]
- Substitution pharmacotherapies for opioid addiction: From methadone to LAAM and buprenorphine. J Psychoactive Drugs. 1994;26:119-28.
- [Google Scholar]
- Tramadol versus buprenorphine for the management of acute heroin withdrawal: A retrospective matched cohort controlled study. Am J Addict. 2006;15:186-91.
- [Google Scholar]
- Diversion of methadone and buprenorphine by patients in opioid substitution treatment in Sweden: Prevalence estimates and risk factors. Int J Drug Policy. 2015;26:183-90.
- [Google Scholar]
- A review of buprenorphine diversion and misuse: The current evidence base and experiences from around the world. J Addict Med. 2014;8:315-26.
- [Google Scholar]
- Delivery models of opioid agonist maintenance treatment in South Asia: A good beginning. Bull World Health Organ. 2013;91:150-3.
- [Google Scholar]
- Barriers to opioid substitution treatment access, entry and retention: A survey of opioid users, patients in treatment, and treating and non-treating physicians. Eur Addict Res. 2011;17:44-54.
- [Google Scholar]
- Efficacy of extended-release tramadol for treatment of prescription opioid withdrawal: A two-phase randomized controlled trial. Drug Alcohol Depend. 2013;133:188-97.
- [Google Scholar]
- Tramadol: A good option for management of opioid withdrawal syndrome in developing countries. J Subst Use. 2016;21:339-40.
- [Google Scholar]
- Tramadol dependence: A case series from India. Indian J Psychol Med. 2012;34:283-5.
- [Google Scholar]
- Effects of repeated tramadol and morphine administration on psychomotor and cognitive performance in opioid-dependent volunteers. Drug Alcohol Depend. 2010;111:265-8.
- [Google Scholar]






