Translate this page into:
Intracranial Germ Cell Tumors: Spectrum of Disease in an Indian Cohort and Management Strategies
Address for correspondence: Dr. Arivazhagan Arimappamagan, Department of Neurosurgery, National Institute of Mental Health and Neurosciences, Bengaluru, Karnataka, India. E-mail: arivazhagan.a@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Germ cell tumors (GCTs) represent approximately 3% of primary pediatric brain tumors in the West, whereas in Asia, they constitute between 8% and 15% of pediatric brain tumors.
Methods:
We retrospectively studied all patients with intracranial GCT managed at our institute from January 1998 to December 2013. The clinical data and radiological data were analyzed.
Results:
Forty-eight patients with intracranial GCT including 36 males and 16 females formed the cohort. The proportion of GCT in our study was 0.29%. The mean age was 16.5 ± 2.5 years. Germinomas constituted 56.3% and nongerminomatous GCTs constituted 43.7% of all the tumors. The most common location was posterior third ventricle (58.3%) followed by suprasellar (22.9%). Histopathological diagnosis was obtained in almost all patients (96%). Surgical procedures included tumor decompression (71.7%), stereotactic biopsy (13%), and endoscopic third ventriculostomy and biopsy (15%). Patient's age, location of the tumor, and histology did not influence the survival. Women with GCTs had poorer survival when compared to men.
Conclusions:
The present study documented a lower hospital-based incidence of GCT in Indian cohort. A multidisciplinary approach including surgical strategy based on location, appropriate radiation planning, and chemotherapy is needed for effective treatment and improved outcomes.
Keywords
Germ cell tumor
germinoma
incidence
management
surgery
INTRODUCTION
Germ cell tumors (GCTs) are tumors of pluripotent cells derived from all the three germ cell layers and recapitulate normal organogenesis. The central nervous system (CNS) is the second most common site of extragonadal GCTs, following mediastinum. CNS GCTs represent approximately 3% of primary pediatric brain tumors in the West, whereas in Asia, they constitute between 8% and 15% of pediatric brain tumors (from series in Taiwan, Japan, and Korea) and encompass a wide pathologic spectrum.[123456]
CNS GCTs are divided into major groups including germinoma, nongerminomatous GCTs (NGGCTs), and teratomas. Neuroimaging studies fail to accurately differentiate GCTs from other tumors, and therefore, the diagnosis usually requires histologic confirmation. In some cases, characteristic elevations of tumor markers, including alpha-fetoprotein (AFP) and/or beta-human chorionic gonadotropin (beta HCG) in the serum and/or cerebrospinal fluid (CSF), may be diagnostic in themselves without the need for tissue confirmation.
The standard management for CNS GCTs remains controversial. The data regarding the GCTs and their proportion among all brain tumors in Indian population are sparse. Two reports have described teratomas in CNS, which included both cranial and spinal location and discussed their management strategies.[78] Recently, a multi-institutional study, comprising the data from three institutions in India, reported that the GCT accounted for 0.43% of CNS tumors.[9] Further, a review of management strategies of these tumors over a long time and their evolution will aid in better management of these tumors. The present study, the largest single institutional study from India, to the best of our knowledge, attempts to document the proportion of GCTs managed in a tertiary neurosurgical institute over a period of 16 years, identify the pathological spectrum, and correlate clinically and elaborate on the management strategies of these patients. These tumors are uncommon, require multidisciplinary approach for management, and pose difficult questions in treatment in view of varied locations and varied histology.
MATERIALS AND METHODS
This retrospective study included patients with proven diagnosis of intracranial GCTs managed at our institute from January 1998 to December 2013. Medical records of patients, who were identified from the histopathology register from the Department of Neuropathology, were reviewed. Some were identified by disease code which included the patients of GCTs who were diagnosed by serum/CSF markers and planned for adjuvant therapy without a histological diagnosis. The spinal GCT cases were excluded from the study. The most recent follow-up available and the present clinical status of the patients were ascertained. The follow-up of the patients was updated as part of the routine management. The percentages were compared using the Chi-square test. P < 0.05 was considered statistically significant. Survival analysis was performed using Log-rank test and Kaplan–Meier curves were obtained.
RESULTS
This study included 48 patients with intracranial GCTs managed during the study period. A total of 16,186 intracranial tumors were operated in our institute from 1998 to 2013, of which 48 were diagnosed as GCTs. The proportion of GCTs in our study was 0.29%. The yearly proportion of cases was 0.27% in the first half, which increased up to 0.4% in the last half of the study [Figure 1].
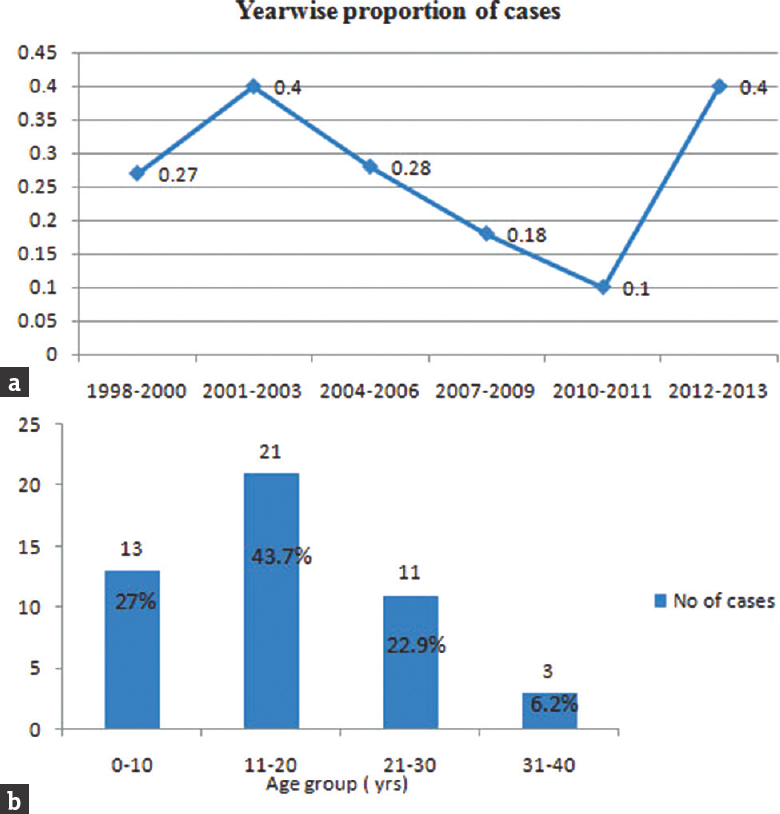
- (a) Graph demonstrates the year-wise proportion of germ cell tumor (percentage) to all intracranial tumors. (b) The distribution of patients with germ cell tumor based on age groups (years)
The year-wise analysis of cases showed a variation in the number of cases from 2001 to 2011 followed by a significant increase in the incidence during the past 2 years (2012–2013). The demographic and clinical details of the study group are shown in Table 1. Germinomas constituted 56.3% and NGGCTs constituted 43.7% of all the tumors. The various salient histological characteristics of GCT are shown in Figure 2. The distribution of histological subtypes in the cohort is given in Table 2. Majority of GCTs (43.7%) were observed in the second decade, whereas the incidence in the first and third decade was comparable (27% and 23%, respectively).
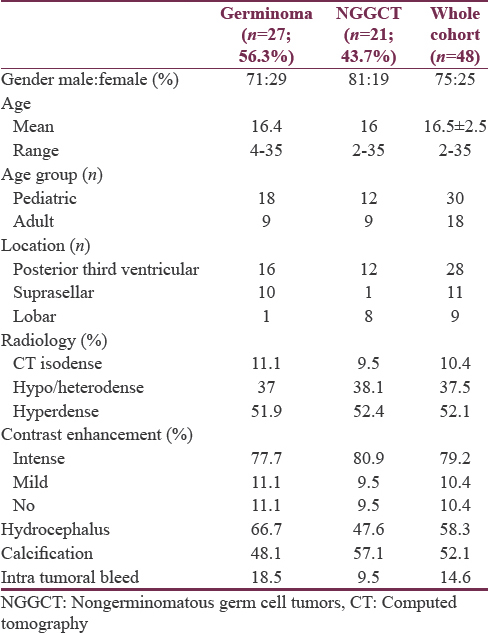
![Montage representing microphotographs of the various Germ Cell Tumors. (a) Germinoma showing sheets of large cells with clear cytoplasm and prominent nucleolus in some cells and intervening lymphocytic infiltrates. (b) Germinoma cells show cytoplasmic staining for PLAP. (c) Mature teratoma composed of mature epidermal, dermal and cartilaginous elements. (d) Immature teratoma- showing immature glandular structures dispersed on a embryonal mesenchymal stroma containing foci of immature cartilage. (e) Immature glands staining positively for α feto protein. (f) Mixed GCT- showing germinoma (left half of panel) and teratoma (right half of panel). (g) Embryonal carcinoma-composed of large epithelial cells arranged in sheets and with macronuclei and brisk mitosis. (h) the cells stain positively for cytokeratin. (i) Yolk sac tumor showing ill-formed Schiller Duval body. (J) Choriocarcinoma– showing cytotrophoblast and syncitiotrophoblastic cells [All images are original magnification x160]](/content/150/2018/9/3/img/JNRP-9-291-g003.png)
- Montage representing microphotographs of the various Germ Cell Tumors. (a) Germinoma showing sheets of large cells with clear cytoplasm and prominent nucleolus in some cells and intervening lymphocytic infiltrates. (b) Germinoma cells show cytoplasmic staining for PLAP. (c) Mature teratoma composed of mature epidermal, dermal and cartilaginous elements. (d) Immature teratoma- showing immature glandular structures dispersed on a embryonal mesenchymal stroma containing foci of immature cartilage. (e) Immature glands staining positively for α feto protein. (f) Mixed GCT- showing germinoma (left half of panel) and teratoma (right half of panel). (g) Embryonal carcinoma-composed of large epithelial cells arranged in sheets and with macronuclei and brisk mitosis. (h) the cells stain positively for cytokeratin. (i) Yolk sac tumor showing ill-formed Schiller Duval body. (J) Choriocarcinoma– showing cytotrophoblast and syncitiotrophoblastic cells [All images are original magnification x160]

Posterior third ventricle was the most common location (58.3%), followed by suprasellar (22.9%) and lobar (16.7%) [Table 1]. Germinomas were most commonly observed in posterior third ventricle (59.3%). Nearly 90.9% (10/11) of tumors in the suprasellar location were germinoma; on the contrary, most of the lobar tumors (88.9%; 8/9) had NGGCT histology.
Clinical features
Patients with GCT presented most commonly with raised intracranial pressure (ICP) (95.8%), followed by visual blurring (85.4%), motor weakness (8.3%), and endocrine disturbances such as diabetes insipidus (DI) (6.3%) and precocious puberty (2.1%). Two patients presented with poor sensorium (Glasgow Coma Scale of 5/15). Patients with suprasellar tumors had loss of visual acuity in 73% of patients, 90% had papilledema, 9% had Parinaud's syndrome, 61% had hemianopia, and 11% had optic atrophy. The radiological characteristics are tabulated [Table 1]. Majority of the patients (87.5%) had a single lesion at initial presentation, while six patients had multiple lesions (12.5%).
Hormonal study
Hormonal studies were available in a total of thirty patients (germinomas: 17 and NGCTs-13). In germinoma patients, serum AFP levels and serum beta-HCG levels were normal in 95% and 76.5% of the patients, respectively. Four patients with germinoma who had an elevated serum beta-HCG level were found to harbor syncytiotrophoblastic component on histopathological examination. CSF AFP levels were not raised in three patients in whom data were available. CSF beta-HCG was found elevated in one patient. In NGGCTs, serum AFP levels were increased in four patients (two each in mixed GCT with yolk sac component and immature teratomas). CSF AFP, available in two patients, was elevated in a patient with mixed GCT. CSF placental alkaline phosphatase was elevated in one patient with mixed GCT.
Metastasis
Five patients (10.5%) had metastatic tumors. The most common primary site was testis. Ovary and liver were the other primary sites. Choriocarcinoma and mixed GCTs constituted 40% each (2 cases each) of the metastatic tumors. Germinoma was observed in one patient (20%). Almost 80% of metastases were multiple and 20% were single lesions.
Treatment
Our institute practice for management comprises that patients with lesions, radiologically suspected to be GCT, will undergo serum hormonal markers. If a CSF diversion procedure was performed, then CSF markers also will be performed. If hormonal markers are noncontributory, then histopathological diagnosis was obtained. In this series, two patients were managed with only CSF diversion followed by adjuvant therapy based on hormonal markers. The surgical procedures, tailored based on the location of tumor and presence of hydrocephalus, included tumor decompression (71.7%; n = 35), stereotactic biopsy (13%; n = 6), and endoscopic third ventriculostomy (ETV) and biopsy (15%; n = 7).
Hydrocephalus was managed with CSF diversion in 29 (72.5%) patients which included ventriculoperitoneal shunts (VPS) (n = 22) or ETV (n = 7). In posterior third ventricular tumors, 18 (64%) underwent decompressive surgery. Among these, Poppen's approach was used in 13 cases and 5 cases underwent Krause's approach. Most of the patients with suprasellar tumors (9/10) underwent transcranial subfrontal/transsylvian decompression. Nearly 75% of lobar tumors underwent surgical decompression. Postoperative complications included hemiparesis (n = 3) and DI (n = 2). One patient with yolk sac tumor involving anterior skull base died within 2 h of the surgical procedure, secondary to excessive intraoperative blood loss.
A proportion of patients only had received radiotherapy (64.5%), usually with the dose of 45 Gy to the tumor bed and 30 Gy of craniospinal irradiation (CSI). This comprised 18 patients with germinoma (66.66%) and 13 patients with NGGCTs (61.9%). Chemotherapy was received by 33.8% patients (four patients with germinoma and four with NGGCT). All patients received a regimen of cisplatin, etoposide, and ifosfamide.
Recurrence was documented in five patients (10.4%) during follow-up, all of which occurred in NGGCTs. All these patients underwent repeat surgery due to significant raised ICP. The histology of recurrence was the same as the initial tumor in all five patients. Recurrence occurred in the same site in four cases, while one patient had a different site of recurrence. The mean duration of recurrence was 6 ± 1.5 months (range: 2–6 months).
Survival
Data regarding survival were available in 41 patients (85.41%) (germinoma – 25 and NGGCTs – 16). Twelve patients had died during the follow-up period (29%). Since the number of events (deaths) was not statistically adequate to calculate median survival values in Kaplan–Meier analysis, mean survival values are mentioned. The follow-up period ranged from 1 to 166 months (mean: 34.5 months). The mean overall survival (OS) for the whole cohort was 110.2 months (95% confidence interval: 84–136.4 months). Various clinical factors and histopathological tumor subtypes were analyzed to evaluate their role in influencing survival [Table 3 and Figure 3]. Clinical variables such as age group and location of tumor did not influence survival. Interestingly, males had a slighter better survival than females, trending toward significance (P = 0.089). The survival was calculated for germinoma, NGGCTs, and teratomas as separate groups. The follow-up in the cases of teratoma was short and none of the patients with teratoma had expired during the follow-up; hence, mean survival could not be calculated for this subgroup. Although the mean OS for germinoma patients was longer than those with NGGCTs, it was not statistically significant in the present study (P = 0.934).
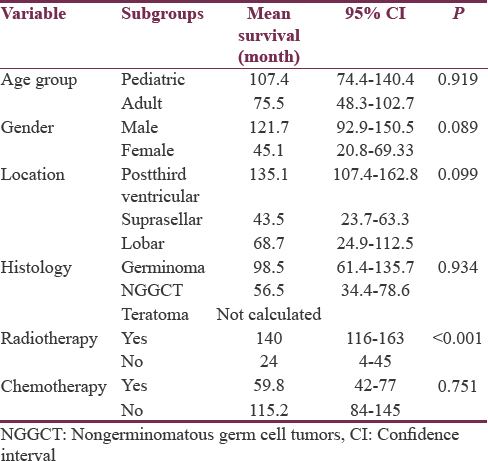
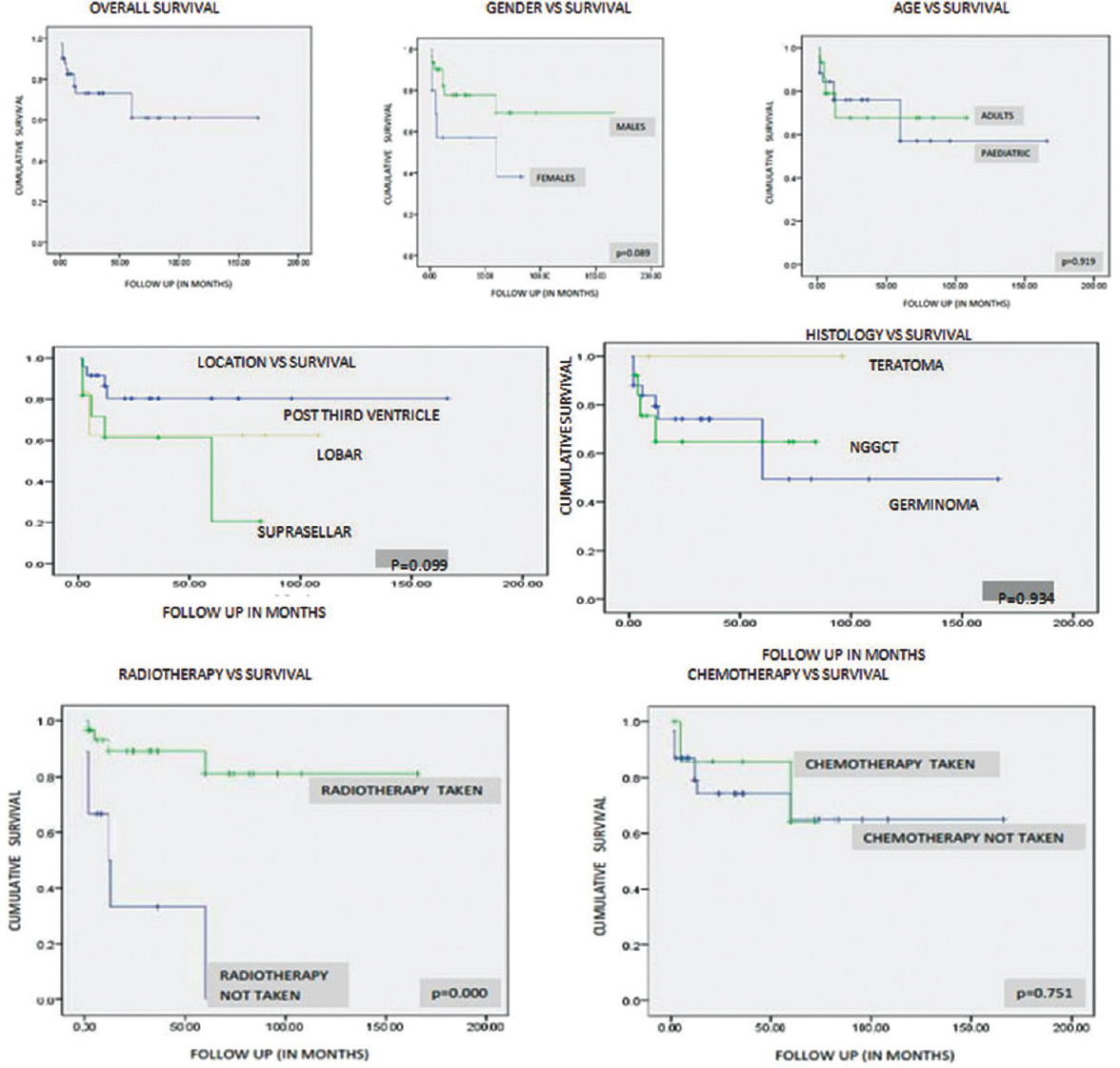
- The Kaplan–Meier survival curves for different variables influencing survival in germ cell tumor
Radiotherapy significantly influenced the survival among patients with GCTs (P < 0.001). This underscores the effect of radiotherapy on GCTs; however, as the majority of the other patients had not received any adjuvant therapy (defaulted the treatment), this result needs to be interpreted with caution. Only eight patients received chemotherapy. Majority of the patients who did not receive chemotherapy have been treated by radiotherapy alone (23/31), which explains the longer survival in this group. Chemotherapy did not influence survival independently (P = 0.751).
DISCUSSION
The incidence of GCTs has been considered to be higher in East Asia than in the Western population. GCTs constitute about 3% of all primary intracranial tumors in the West. In the Oriental countries, the incidence rises to about 9%–15%. Institutional comparisons can provide insightful direction in the management of such conditions. The data regarding the GCTs and their proportion among all brain tumors in our population are incomplete. Our study documented that intracranial GCTs constitute about 0.29% of all the intracranial tumors managed in our institute over a long period of 16 years. Similar incidence (0.43%) has been recently reported by Kakkar et al. from another multi-institutional study in India.[9]
The yearly proportion of cases over a period of 16 years was about 0.27%. These findings vary considerably as compared to Caucasian and oriental population. GCTs represent higher proportions of brain tumors in Japan (7.8%), Korea (11.2%), Taiwan (14.0%), and China (7.9%), whereas in the United States, Canada, and Europe, frequencies vary between 1% and 3%.[12345] However, in the largest international comparison study reviewing four tumor databases by McCarthy et al., there is a negligible difference in the incidence of primary GCTs in the USA and Japan (0.1 and 0.17 per 105 population, respectively).[5]
CNS GCTs occur mostly in the young population with approximately 90% of the cases before the age of 20 years. Villano et al. reported mixed germ-cell tumors with the lowest mean age at onset of 14.3 years and germinomas with a mean age of 19.2 years in the SEER database.[10] In both CBTRUS and NCDB data, teratomas had the lowest mean age at diagnosis (10.5 and 11.6 years, respectively). Germinomas had a mean age at diagnosis of 20.2 years in the NCDB database, similar to the SEER data.
In our study, there was a male predominance with 75% being males. Villano et al. in their review of three databases showed a male predominance for malignant germ-cell tumors, with the male:female ratios ranging from 14.3:1 to 21.4:1.[10] Rosenblum and Matsutani have described an estimated male-to-female ratio of 3:1 in NGGCTs and about 1.8:1 in germinomas.[11]
The location of the lesion appears to be influenced by gender in GCTs. Jennings et al. have reported that, in males, 70% of tumors occur in the pineal region, and in females, 75% of tumors are suprasellar.[12] McCarthy et al. reported male-to-female incidence of 11.5:1 for posterior third ventricular tumors as compared to 1.9:1 for tumors located in the nonpineal region in the Japanese population. Similarly, there was a male preponderance in the pineal region tumors with an incidence of 16.0:1, as opposed to 2.1:1 for tumors located in the nonpineal region.[5] In concurrence, we noted that all posterior third ventricular GCTs were observed in males and none in females. Similarly, females constituted 72.7% of GCTs in suprasellar region.
Germinomas are usually more common than NGGCTs, as also reflected in our study. Germinomas constituted 60% of the posterior third ventricular/pineal tumors, whereas NGGCTs were observed in 40% and are comparable with current literature. McCarthy et al. reported that germinomas were observed in 82% of patients in Japanese group and 77.5% in Caucasians.[5] Villano et al. studied three tumor registries. In the SEER database, germinomas made up the greatest proportion of pineal region germ-cell tumors (73.0%). Similarly, in the CBTRUS and NCDB data sets, germinomas comprised the greatest proportion of pineal GCTs (85.7% and 80.9%), respectively.[10] In our study, mixed GCTs were observed in 18.8% of patients and teratomas in 6.4% of patients. Among the NGGCTs, the most common histology was mixed GCT. In the mixed GCT, immature teratoma component was observed more frequently (33.33%).
The current treatment for CNS GCTs includes a combination of tumor resection, RT to the tumor cavity to control tumor, and CSI to treat/prevent the involvement of leptomeninges. Chemotherapy is also used to prevent leptomeningeal and systemic tumor dissemination. Neurosurgical intervention is required for diagnostic sampling, tumor cytoreduction, and/or hydrocephalus treatment.
McCarthy et al. reported that almost half (48%) of all GCTs and 56% of germinomas did not receive surgical treatment as the first course of therapy.[5] However, the Japanese database according to the BTRJ showed that 62% of all GCTs and 63% of germinomas received surgical treatment consisting of biopsy, local or partial removal, while less than one-third of tumors were not treated with a surgical procedure as the first course of treatment.[5] The role of surgical resection, both total and near-total resection, is unproven in germinoma, but it is beneficial in obtaining disease control for NGGCTs, while it could be curative for teratomas.[13] Kamoshima and Sawamura have reported that ETV is preferred over VPS as it simultaneously allows tumor biopsy as well as improvement of hydrocephalus.[13] Ray et al., in a retrospective review of 43 patients and at a mean of 2-year follow-up, reported a 70% success rate using ETV instead of VPS.[14] In our series, histopathological diagnosis was obtained in almost all patients (96%), with only two patients in the study group managed with adjuvant therapy based on hormonal markers. This variation needs to be taken with caution, as there is a likely possibility that some patients have not been included who were diagnosed based on hormonal markers in our series, this being retrospective and largely based on histopathology database.
In the NCDB, 68% of all GCTs and 74% of germinomas received radiation treatment as a first course of therapy.[5] All patients who received radiotherapy in our study received a dose of 45 Gy to the tumor bed and 30 Gy of CSI. This was in accordance to most studies where doses of primary site irradiation have ranged between 4000 and 5500 cGy.[15161718] About 33.8% patients received chemotherapy. In the NCDB database, chemotherapy was used as the first course of treatment in 46% of germinomas and 48% of all GCTs.[5]
Germinomas carry an excellent prognosis, with most series suggesting 5-year progression-free survival rates and cure in over 90% of patients.[19] However, NGGCTs, including mixed GCTs and embryonal cell carcinomas or yolk sac tumors, have a poorer prognosis, with reported survival rates ranging from 40% to 70%.[172021]
In our series, we noted that the patient's age, location of the tumor, and histology of the tumor did not influence the survival significantly. The mean survival of germinoma was longer than that of NGGCT, which however, did not reach statistical significance. While suprasellar tumors demonstrated poorer survival compared to the tumors in other locations, the difference was not statistically significant. We noted that women with GCTs had poorer survival when compared to men, with the difference in survival tending toward statistical significance.
The survival in germinoma in our series was less as compared to that published by McCarthy et al.[5] (cumulative survival estimates for germinomas were over 81% at 5 years in both datasets) and Matsutani et al., who reported that the 5-, 10-, 15-, and 20-year survival rates in patients with germinomas were 95.4%, 92.7%, 87.9%, and 80.6%, respectively, whereas the 5- and 10-year survival rates in patients with mixed tumors were 57.1% and 40.1%, respectively.[22]
The survival was significantly high in patients who received radiotherapy with 87% of patients receiving radiotherapy being alive as compared to 33% who did not receive radiotherapy. However, chemotherapy as an independent factor did not significantly influence the survival. This underscores the importance and the efficacy of adjuvant therapy in the management of this complex and histologically diverse tumors. None of the patients with teratoma in our cohort have expired, which also highlights the fact that though they are grouped under NGGCTs, the survival of teratomas needs to be considered separately to portray a clear picture about survival rates based on histology.
CONCLUSIONS
The present study documented a lower hospital-based proportion of GCT in Indian cohort. The patient's age, location of the tumor, and histology did not influence the survival. Women with GCTs had poorer survival when compared to men. A multidisciplinary approach including surgical strategy based on location, appropriate radiation planning, and chemotherapy is needed for effective treatment and improved outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Brain tumors in childhood: Statistical analysis of cases from the brain tumor registry of Japan. Childs Nerv Syst. 1986;2:233-7.
- [Google Scholar]
- Time trends and characteristics of childhood cancer among children age 0-14 in Shanghai. Pediatr Blood Cancer. 2009;53:13-6.
- [Google Scholar]
- Pediatric brain tumors: Statistics of SNUH, Korea (1959-2000) Childs Nerv Syst. 2002;18:30-7.
- [Google Scholar]
- Primary pediatric brain tumors: Statistics of Taipei VGH, Taiwan (1975-2004) Cancer. 2005;104:2156-67.
- [Google Scholar]
- Primary CNS germ cell tumors in Japan and the United States: An analysis of 4 tumor registries. Neuro Oncol. 2012;14:1194-200.
- [Google Scholar]
- Epidemiology of nervous system tumors in children: A survey of 1,485 cases in Beijing tiantan hospital from 2001 to 2005. Pediatr Neurosurg. 2008;44:97-103.
- [Google Scholar]
- Intracranial teratomas in children: A clinicopathological study. Childs Nerv Syst. 2013;29:2035-42.
- [Google Scholar]
- Teratomas in central nervous system: A clinico-morphological study with review of literature. Neurol India. 2010;58:841-6.
- [Google Scholar]
- Intracranial germ cell tumors: A multi-institutional experience from three tertiary care centers in India. Childs Nerv Syst. 2016;32:2173-80.
- [Google Scholar]
- Malignant pineal germ-cell tumors: An analysis of cases from three tumor registries. Neuro Oncol. 2008;10:121-30.
- [Google Scholar]
- CNS germ cell tumours. In: Kleihues PC, ed. Pathology and Genetics of Tumours of the Nervous System. Vol 2. Lyon, France: International Agency for Research on Cancer; 2000. p. :208-14.
- [Google Scholar]
- Intracranial germ-cell tumors: Natural history and pathogenesis. J Neurosurg. 1985;63:155-67.
- [Google Scholar]
- Update on current standard treatments in central nervous system germ cell tumors. Curr Opin Neurol. 2010;23:571-5.
- [Google Scholar]
- Endoscopic third ventriculostomy for tumor-related hydrocephalus in a pediatric population. Neurosurg Focus. 2005;19:E8.
- [Google Scholar]
- Histologically confirmed pineal tumors and other germ cell tumors of the brain. Cancer. 1996;78:2564-71.
- [Google Scholar]
- Pineal region germinomas in childhood treatment considerations. Int J Radiat Oncol Biol Phys. 1990;18:541-5.
- [Google Scholar]
- Radiation therapy for histologically confirmed primary central nervous system germinoma. Int J Radiat Oncol Biol Phys. 1997;38:915-23.
- [Google Scholar]
- Epidemiological survey of central nervous system germ cell tumors in Canadian children. J Neurooncol. 2007;82:289-95.
- [Google Scholar]
- Germ cell tumours of the central nervous system: Treatment consideration based on 111 cases and their long-term clinical outcomes. Eur J Cancer. 1998;34:104-10.
- [Google Scholar]
- Primary intracranial germ cell tumors: A clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446-55.
- [Google Scholar]






