Translate this page into:
Intracranial collision tumor: A rare case report
*Corresponding author: Shruti Gupta, Department of Pathology, All India Institute of Medical Sciences, Raebareli, Uttar Pradesh, India. drshrutimlb@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Biswas M, Gupta S, Kumari N, Keelara AG, Suman AK, Singh S. Intracranial collision tumor: A rare case report. J Neurosci Rural Pract. doi: 10.25259/JNRP_349_2024
Abstract
Collision tumors yield two distinct histopathological entities in the same anatomic location and are rarely encountered in routine neuropathology practice. The review of literature has revealed only 33 cases of collision tumor. We aim to report a case of an intracranial collision tumor in a 53-year-old male patient with no prior history or known symptoms of a pre-existing malignancy and who succumbed to the disease. The histopathology revealed both glial and non-glial tumor: Isocitrate dehydrogenase-mutant astrocytoma (World Health Organization Grade 4) and metastatic adenocarcinoma. The lack of awareness and resources in rural patients regarding symptomatology of cancers can lead to reduced index of suspicion for pre-existing malignancies. This case highlights the need for adequate pre-operative systemic and radiological examination and meticulous histopathological evaluation for accurate diagnosis and management.
Keywords
Astrocytoma
Collision tumor
Intracranial
Metastatic carcinoma
INTRODUCTION
A collision tumor, a rare scenario, by definition, is the cooccurrence of two entirely different neoplasms at the same anatomic location.[1] It may develop in different areas of the body including the thyroid gland,[2] ovary,[3] kidney,[4] and the gastrointestinal system.[5] Intracranial occurrence of collision tumors is a rare phenomenon in routine neurosurgery practice and in most of the cases reported in the literature, the components are primary brain tumors.[6] The presence of cerebral metastasis as a component of intracranial collision tumor is extremely rare.[7] Thus, we report a case of a 53-year-old male with an intracranial collision tumor showing both glial and non-glial tumors: Isocitrate dehydrogenase (IDH)-mutant astrocytoma (World Health Organization [WHO] Grade 4) and metastatic adenocarcinoma.
CASE REPORT
A 53-year-old gentleman presented to the neurosurgery outpatient department with complaints of slurring of speech for 1 month and pain and weakness in the right upper and lower extremities for the past 15 days. There was no history of any loss of consciousness, vomiting, seizure, or headache. He had no evidence of any other chronic disease that may lead to neuro-deficit. The patient did not describe any symptoms or diagnosis of any malignant or pre-malignant condition in the past. On neurological examination, he had grossly reduced power over the right upper limb. The general clinical systemic examination was unremarkable. A contrast-enhanced magnetic resonance imaging scan of brain revealed mass lesions at the left parietal lobe and left cerebellar hemisphere. Serum CA-19-9 and carcinoembryonic antigen were elevated; however, no further investigations were done in this regard. After pre-operative evaluation of the brain lesion, left parietal craniotomy and excision of the mass lesion were performed. Intraoperatively, the mass was soft to firm, suckable, and associated with excess bleeding. The specimen was sent for histopathological examination. The post-operative hospital course was uneventful. The patient was discharged and advised for a follow-up visit.
Grossly multiple, fragmented, gray-white soft tissue pieces altogether measuring 2 × 2 × 0.7 cm were received for histopathology. All the tissue pieces were embedded. On microscopic examination, sections showed two distinct regions showing varied tumor morphology on low power. One area showed a hypercellular glial tumor composed of hyper-chromatic and mild pleomorphic cells with endothelial proliferation and few mitotic figures suggesting a high-grade glioma [Figure 1a-c], while other foci showed a nonglial tumor with epithelial differentiation, arranged in a glandular pattern and nest, having markedly pleomorphic, hyperchromatic nuclei with prominent nucleoli and moderate cytoplasm. Immunohistochemistry done on morphological high-grade glioma showed diffuse immunopositivity for glial fibrillary acidic protein (GFAP), cytoplasmic positivity for IDH, and nuclear positivity for p53. ATRX was retained. Ki67 proliferation index was 70–80% [Figure 1d-f]. Brisk mitotic figures were noted along with areas of necrosis [Figure 2a-c]. Normal glial tissue was also identified in the adjacent area. Based on the above morphology, a diagnosis of a collision tumor with high-grade glioma and metastatic carcinoma was offered. As advocated by the latest WHO classification of central nervous system tumors,[8] immunohistochemical markers for the glioma panel were done. Depending upon the morphological features and the immunohistochemistry, the final diagnosis was made as IDH-mutant astrocytoma (WHO Grade 4). The epithelial deposits were immune-negative for GFAP.
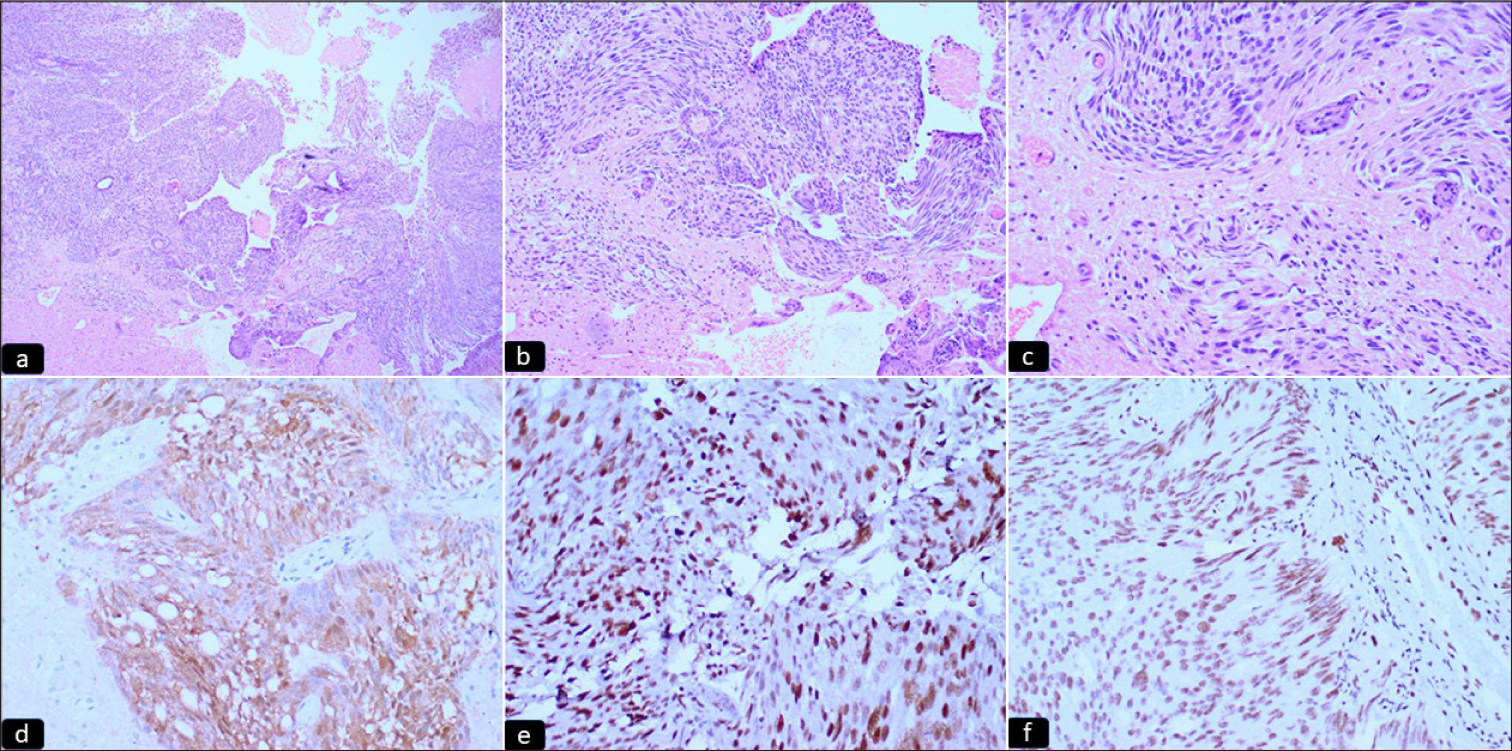
- (a-c) Microphotograph sections showing a hypercellular glial tumor with prominent endothelial proliferation (hematoxylin and eosin 4X, 10X, 20X, respectively). (d-f) Microphotograph sections showing immunohistochemical expression of (d) IDH (e) p53 and (f) ATRX. The tumor cells show immunopositivity for IDH and p53 and ATRX expression is retained, diagnostic of IDH-mutant Grade 4 astrocytoma (IPOX) (hematoxylin and eosin 20X).
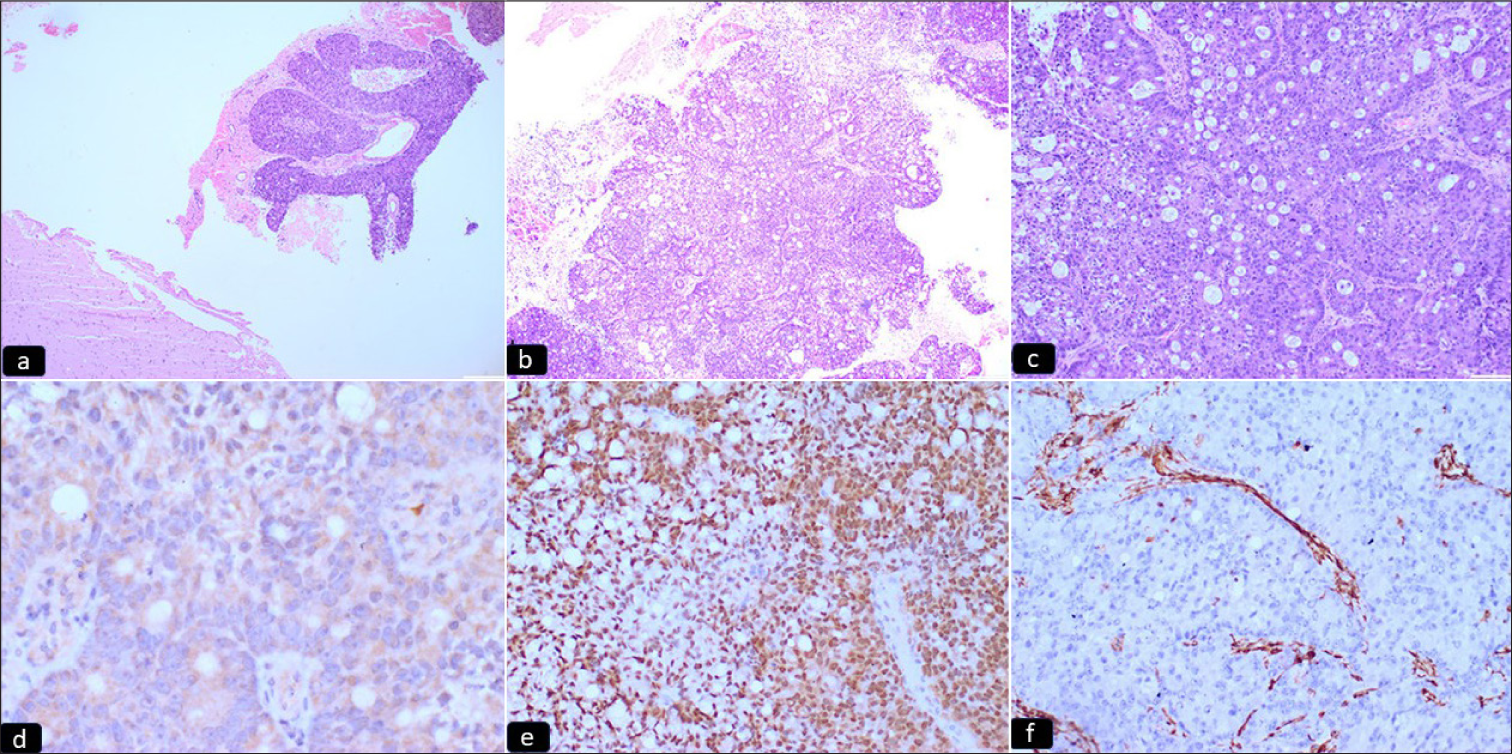
- (a-c) Microphotograph sections showing a non-glial tumor with epithelial differentiation, arranged in glandular pattern and solid sheets. Normal glial tissue is noted lying adjacent (hematoxylin and eosin 4X, 10X, 20X, respectively). (d-f) Microphotograph sections showing immunohistochemical expression of (d) CK20, (e) CDX2, and (f) Vimentin. The tumor cells show immunopositivity for CK20 and CDX2, indicative of gastrointestinal origin (IPOX) (hematoxylin and eosin 20X).
Simultaneously, to evaluate the carcinoma of unknown primary, a panel of markers was done. The tumor cells showed membranous positivity for CK7; cytoplasmic positivity for CK20 (Focal-10%); nuclear positivity for CDX2; and immuno-negativity for thyroid transcription factor-1, CD10, and vimentin in metastatic tumor cells, suggesting metastatic adeno-carcinomatous deposit possibly of gastrointestinal origin [Figure 2d-f]. The clinician was advised to evaluate the patient for the site and extent of gastrointestinal malignancy. However, the patient was lost to follow-up and expired 4 months after the surgery due to unknown causes.
DISCUSSION
Collision tumors yield two distinct histopathological entities in the same anatomic location.[1] Another terminology reported in the literature is tumor-to-tumor metastasis, where hematogenous metastatic cancer inoculates in the primary tumor mass.[9] However, these two terminologies are sometimes used interchangeably in the literature. The exact pathophysiology for the development of collision tumors is unknown although some hypotheses are postulated. Collision tumor may develop due to the occurrence of two distinct tumor entities by coincidence.[10] It may also develop as a result of malignant transformation of the surrounding brain tissue following radiation therapy, prior surgery, or trauma.[10] Zhang et al. have suggested it to be a dynamic process where one tumor develops later in close proximity to the first tumor.[10] An assessment of series of collision tumors can provide thorough understanding into the pathophysiology of collision tumors. A long-term follow-up and syndromic associations of synchronous malignancies need to be studied in detail for any prognostic implications in these patients.
Intracranial collision tumor is rare; mostly comprising of two primary tumors with meningioma being the most commonly reported entity.[7] Collision tumors comprising meningioma in combination with glioma, pituitary adenoma, and schwannoma have been reported.[11-13] Secondary intracranial metastasis is rarely documented as a component of intracranial collision tumor. The metastasis may be from the lungs, breast, kidney, genito-urinary tract, and rarely the gastrointestinal tract. Although brain tumors are primarily diagnosed by computed tomography and magnetic resonance imaging (MRI), the diagnosis of intracranial collision tumors is difficult with these imaging modalities. It is purely a histopathological diagnosis. Here, we present a case where on contrast-enhanced MRI scan, there was a mass lesion at the left parietal lobe and left cerebellar hemisphere. We found a high-grade glial tumor in the left parietal lobe coexisting with a focus of metastatic adenocarcinoma possibly of gastrointestinal origin. The high-grade glial tumor was further documented as IDH-mutant astrocytoma (WHO Grade 4) after immunohistochemical examination. These conditions may pose a challenge to the treating surgeon as well as to the pathologist. To our knowledge, this case counts among the handful of cases reported as intracranial collision tumors in the world literature.
An extensive search of the PubMed database for intracranial collision tumors yielded 33 published articles with 39 reported cases [Supplementary Table 1]. Out of them, 14 articles were published in the USA, followed by China (3 articles), Greece, and India (each with 2 articles). The mean age of the reported cases is 50.02 years with a standard deviation of 20.47. Male and female patients were almost equal in numbers (Male - 20, female - 19). The most common location was reported as the frontal lobe (16 cases), followed by the parieto-occipital region (5 cases) and the cerebellum (3 cases). The most common primary tumor as a component of intracranial collision tumor was reported as meningioma (26 cases), followed by glioblastoma (10 cases), astrocytoma (5 cases), and schwannoma (3 cases). Among these 39 cases, metastatic carcinoma was a component of intracranial collision tumor in seven patients. Out of them, two were primarily of lung origin. Other reported origins of metastasis were breast, kidney, uterus, prostate, and esophagus. In our case, the primary origin of the metastatic carcinoma was the gastrointestinal tract without any significant history of the symptoms related to the primary malignant site.
An extensive literature search comprises mostly case reports and does not provide any specific insights regarding any characteristic pre-operative clinical and radiological findings. However, a thorough pre-operative clinical examination must be done in space-occupying brain lesions to rule out any metastatic lesions. Owing to the rarity of collision tumors, standard management guidelines are also not described.
The patient was lost to follow-up and expired, therefore further work-up and investigations could not be done.
CONCLUSION
Intracranial collision tumors are extremely rare and pre-operative diagnosis is usually not possible. However, awareness of this morphological entity is imperative for neurosurgeons and pathologists for accurate diagnosis and management. A detailed review regarding the etiopathogenesis of collision tumors with a metastatic component needs to be done. This review highlights the importance of exhaustive pre-operative clinical examination by neurosurgeons and meticulous histopathological and immunohistochemical evaluation by pathologists. The histopathology diagnosis is definitive and is imperative for accurate staging and further work-up regarding the management of these lesions.
Ethical approval:
Institutional review board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Metastases of central nervous system neoplasms. Case report. J Neurosurg. 1988;68:811-6.
- [CrossRef] [PubMed] [Google Scholar]
- Papillary and medullary thyroid carcinomas presenting as collision tumors: A case series of 21 cases at a tertiary care cancer center. Head Neck Pathol. 2021;15:1137-46.
- [CrossRef] [PubMed] [Google Scholar]
- Collision tumor of the ovary: Fibroma and mature cystic teratoma. Indian J Pathol Microbiol. 2021;64:171-3.
- [CrossRef] [PubMed] [Google Scholar]
- Primary collision tumors of the kidney composed of oncocytoma and papillary renal cell carcinoma: A review. Ann Diagn Pathol. 2017;29:32-6.
- [CrossRef] [PubMed] [Google Scholar]
- Collision tumor: A colonic adenocarcinoma and a gastric adenocarcinoma. Acta Chir Belg. 2022;122:373-6.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative report on intracranial tumor-to-tumor metastasis and collision tumors. World Neurosurg. 2018;116:454-63.e2.
- [CrossRef] [PubMed] [Google Scholar]
- A rare intracranial collision tumor of meningioma and metastatic uterine adenocarcinoma: Case report and literature review. World Neurosurg. 2021;145:340-7.
- [CrossRef] [PubMed] [Google Scholar]
- The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23:1231-51.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor to tumor metastasis: Report of two cases and review of the literature. Int J Surg Pathol. 2003;11:127-35.
- [CrossRef] [PubMed] [Google Scholar]
- An intraventricular meningioma and recurrent astrocytoma collision tumor: A case report and literature review. World J Surg Oncol. 2015;13:37.
- [CrossRef] [PubMed] [Google Scholar]
- Coexisting intracranial tumors with pituitary adenomas: Genetic association or coincidence? J Cancer Res Ther. 2010;6:221-3.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous presentation of meningiomas with other intracranial tumours. Br J Neurosurg. 2005;19:368-75.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic endonasal resection of a synchronous pituitary adenoma and a tuberculum sellae meningioma: Technical case report. Neurosurgery. 2007;60(4 Suppl 2):E401.
- [CrossRef] [PubMed] [Google Scholar]







