Translate this page into:
Impact of Spectral Severity of Alcoholism on Visual-Evoked Potentials: A Neuropsychiatric Perspective
Address for correspondence: Dr. Ruchi Kothari, Department of Physiology, Mahatma Gandhi Institute of Medical Sciences, Sevagram, Wardha - 442 102, Maharashtra, India. E-mail: ruchi@mgims.ac.in
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The deleterious effects of alcohol on the brain are replete in literature. Only a few neurophysiologic measures can pick up the neuronal dysfunctions, one of them being visual-evoked potential (VEP). A very limited amount of data exists on the progression of neural abnormalities related to the spectral severity of alcoholism.
Aim of the Study:
To evaluate the impact of spectral severity of alcoholism through VEP and to understand the emergence of any specific pattern or morphometric abnormalities related to alcohol-induced neuropsychiatric presentations.
Methodology:
A total of 90 cases were recruited in addition to 180 age- and sex-matched controls using purposive and random sampling. The Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version and Campbell Neuropsychiatric Inventory were used to evaluate alcohol disorders and its neuropsychiatric complications apart from the mandatory consultant-specific clinical evaluations of all the cases. Of 90 cases of alcohol dependence, 15 patients were currently abstinent for >6 months, 15 had alcohol intoxication, 15 had signs of alcohol withdrawal, 15 had physical complications, 15 had psychiatric comorbidity, and 15 had neurological complications such as epilepsy. VEP recordings were taken using an Evoked Potential Recorder (RMS EMG. EP MARK II) where the stimulus configuration consisted of transient pattern-reversal method in which a black and white checkerboard was generated full field.
Results:
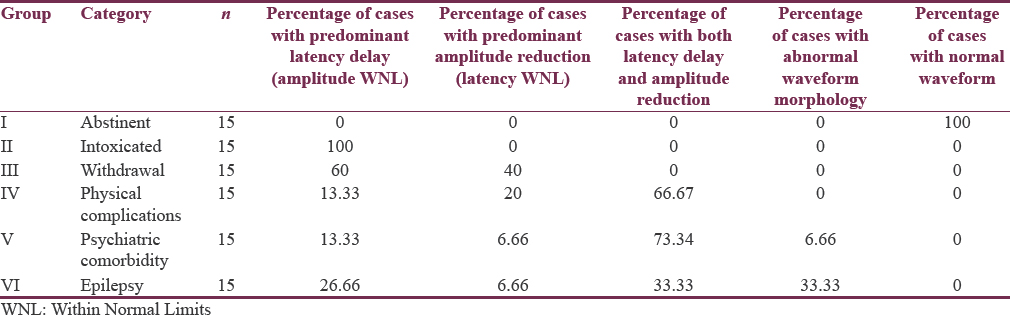
Mean age of cases was 37.71 ± 11.49 years compared to 39.43 ± 10.67 years in controls (range 18–65 years). VEP abnormalities comprising of prolonged latencies (62.5%) with a statistically significant difference (P < 0.001) from the healthy controls was observed in cases of alcohol withdrawal syndrome. Predominant amplitude reduction with normal latency was obtained in 37.5% cases of withdrawal. Severe VEP abnormalities, i.e., both latency delay and amplitude reduction, were found in 75% patients with psychiatric comorbidity, 66.67% patients with neurological complications, i.e., epilepsy, and 33.34% patients with physical complications. An explicit finding of prominent interocular differences was a prominent feature present in 25% of patients with complications.
Keywords
Alcohol dependence
amplitude
latency
neuropsychiatric
psychiatric complications
visual-evoked potential
withdrawal
INTRODUCTION
Alcohol (ethanol) is one of the most widely misused drugs in both developed and developing countries. Neural functional pathologies may exist in alcohol addicts even when no obvious clinical manifestations are apparent. Neural functional pathologies may also exist where fatal complications are evident. In the National Comorbidity Study, 29.2% of respondents with alcohol dependence experienced either an independent or a substance-induced mood disorder within the past 12 months of their assessment, a rate that was 3.9 times higher than those who were not alcohol dependent. Bipolar disorder over the previous year was seen in 1.9% of the respondents with alcohol dependence, a rate 6.3 times greater than nonalcoholics.[123] Thus, factors leading to these observed/induced changes are poorly understood, and there are hardly any ways or means to predict who would develop neural insults and who would not. The question that remains is – If alcohol itself causes insult to the brain and to what extent it can be picked up early? Can we detect or measure the continuity or progressive involvement in any way? The best models used in understanding alcohol-induced brain damage have been retrieved from the studies related to alcohol-induced optic neuropathy, encephalopathy, or seizures. For example, patients with alcohol-related optic neuropathy present with a bilateral, progressive, painless loss of visual acuity, dyschromatopsia, and subsequent disc changes including marked temporal disc pallor and retinal nerve fiber layer loss mainly in the papillomacular bundle. Early detection and prompt management may decrease visual impairment and aid in reversing the optic nerve damage and revival in visual status.[4] It has been achieved clinically with the use of visual-evoked potential (VEP), which serves as an important means of obtaining reproducible, qualitative, and quantitative data on the function of the visual pathways and the visual cortex.[5]
A few studies have reported changes in VEP following alcohol consumption. A study in Spain found that the amplitude of VEP increases with pattern reversal, at a blood alcohol level (BAL) of 0.8 g/kg.[6] In the USA, Krull et al.[7] found that alcohol increases the latency of a 250-ms negative component (N2) only in the absence of sleep deprivation. Other studies found no correlation between the level of alcohol consumption and VEP parameters for the first deflection.[8] Quintyn et al.[9] studied changes in the vision of 16 people after consumption of a small quantity of alcohol. Their results indicated that alcohol consumption caused no significant difference in performance. Further, VEP has also been shown to identify how its specific morphological pattern depending on BAL can help us know the severity of the brain involvement. Visual target detection in a treatment-naïve alcohol-dependent (TNAD) sample versus age and gender comparable nonalcoholic controls was investigated by Fein and Andrew.[10] The significant reduction in P3b amplitude in TNAD reflected the effects of active alcohol abuse.
Petit et al.[11] have concluded that elevated alcohol cue reactivity may lead to poorer inhibitory performance in heavy social drinkers and may be considered as an important vulnerability factor in developing alcohol misuse when assessing event-related potentials (ERPs) in them.
Another ERP study by Petit et al.[12] showed that reduced processing of alcohol cues predicts abstinence in recently detoxified alcoholic patients in a 3-month follow-up period. Abstainers presented with decreased P3 amplitude in this study.
While alcohol dependence is associated with augmented automatic attentional biases early in processing, escape drinking was found to be related with more controlled attentional biases to active alcohol cues during a relatively later stage in processing. This was reported in a recent study by Dickter et al.[13]
Recently, occipital ERPs to addiction-related stimuli in detoxified patients with alcohol dependence and their association with 3-month relapse were studied by Matheus-Roth et al.[14] Their results indicated a sensitivity of occipital ERPs to addiction-related stimuli.
However, scanty data exist for the utility of VEP on the progression of neural abnormalities related to spectral severity of alcoholism when it comes to neuropsychiatric implications.
There is a dire need for a study which can explore comprehensively or conceptually empirical evidence or a neurophysiological biomarker which could help us develop preventive vigilance for identifying those who could develop such neuropsychiatric complications. In this context, the present study aimed at evaluating the impact of spectral severity of alcoholism through VEP to understand the emergence of any specific pattern or morphometric abnormalities related to alcohol-induced neuropsychiatric presentation.
Objectives
-
To screen the patients by both clinical and neurophysiological evaluations for the spectral severity of alcoholism
-
To identify specific patterns emerged between spectral severity and to determine temporal association of VEP abnormalities in patients on spectral severity of alcoholism.
METHODOLOGY
Setting
Outpatient and inpatient services of the Department of Psychiatry, Department of Medicine, and Department of Surgery in our tertiary care rural hospital were the study setting.
Place of the study
Neurophysiology unit, Department of Physiology, Mahatma Gandhi Institute of Medical Sciences, Sevagram, Wardha, Maharashtra, was the place of the study.
Study design
This was a cross-sectional, observational, noninterventional, single-time assessment, hospital-based study.
We used purposive and random sampling to determine the samples.
A total of 90 cases (15 patients each in 6 subgroups) of patients on spectral severity of alcohol use disorder with physical/neuropsychiatric complications and 180 age- and sex-matched controls were recruited after screening 668 patients of alcohol dependence syndrome visiting either to the Department of Psychiatry, Medicine, or Surgery of our tertiary care rural hospital for studying VEP abnormalities.
Ninety cases of alcohol dependence were divided into six following groups:
-
Fifteen patients of alcohol dependence syndrome who were currently abstinent for >6 months
-
Fifteen patients had alcohol intoxication and were under treatment for the last 72 h
-
Fifteen patients who had signs of alcohol withdrawal syndrome and were admitted to psychiatric inpatient services
-
Fifteen patients who had physical complications related to liver (hepatic encephalopathy secondary to alcoholic cirrhosis) and were admitted to inpatient services of either of medicine or surgery
-
Fifteen patients who had psychiatric comorbidity of alcohol-induced mood disorder/psychotic disorder after stopping alcohol for more than a month and were admitted to the inpatient services of psychiatry.
-
Fifteen patients who had neurological complications such as epilepsy induced by alcohol use disorder and who were evaluated just before/within a day of starting antiepileptics.
The healthy 180 controls who were age and sex matched were also screened for any abnormalities before neurophysiological evaluation. All participants reported normal or corrected-to-normal vision, wherever possible, and this was corroborated by medical records. Controls were free of any psychiatric illness or symptoms, and they reported no history of alcohol or substance abuse in the last 5 years or more.
Only those participants who fulfilled the inclusion criteria were selected.
Inclusion criteria
The men aged 18–60 years were included in the study. The patients who were diagnosed as chronic alcoholics according to the strict Diagnostic and Statistical Manual-IV (DSM-IV-TR)[15] criteria developed by the American Psychiatric Association for the clinical diagnosis of abuse and dependence were included. The presence of family history of alcoholism in the first- or second-degree relative and those patients who are physically stable enough to undergo VEP and competent and willing to give informed consent were also included in the study.
Exclusion criteria
The patients with lens/corneal opacities, miotic pupil, and recent eye medications (mydriatics or cycloplegics in the past 12 h); patients with serious systemic illness affecting the performance of VEP, history of other neurological disorder, or heart disease; patients with subnormal intelligence, history of comorbid diagnosed neurological disorders such as epilepsy or neurodegenerative disorders, comorbid substance abuse, except minimal nicotine use; patients with history of head injury or patients having undergone recent neurosurgery, Suicidal/homicidal/catatonic patients, presence of tardive dyskinesia/antipsychotic-induced movement disorders, those not willing for study participation or whosoever refused to be a part of our study; patients with any history of visual impairment beyond corrected-to-normal vision; patients with any uncooperative or febrile patient were not included in the study.
Recruitment/assessment of participants
This was done under two heads namely clinical analysis and neurological evaluation.
Clinical analysis
Sociodemographic profile sheet was filled for each subject, and psychiatric status of alcoholic patients was assessed using Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version[16] and Campbell Neuropsychiatric Inventory for additional neuropsychiatric complications apart from consultant-specific clinical evaluation for all cases were done by a consultant psychiatrist.
Neurophysiological evaluation
All the participants were evaluated for transient pattern-reversal VEP by a neurophysiologist.
Procedure of visual-evoked potential recordings
Transient-pattern reversal VEP (PRVEP) recordings were done in accordance with standardized methodology of the International Federation of Clinical Neurophysiology Committee Recommendations[17] and International Society for Clinical Electrophysiology of Vision Guidelines,[18] and montages are kept as per 10–20 International System of Electroencephalography electrode placements keeping the reference electrode (Fz) placed 12 cm above the nasion, the ground electrode (Cz) at the vertex, and the active electrode (Oz) at approximately 2 cm above the inion.
The electrode impedance was kept below 5 KΩ. The stimulus configuration consisted of the transient pattern-reversal method in which a black and white checkerboard is generated (full field) on a VEP Monitor by an electronic pattern regenerator inbuilt in an Evoked Potential Recorder (RMS EMG. EP MARK II manufactured by Recorders and Medicare Systems, Chandigarh). The rate of pattern reversal (1.7 Hz), the size of the checks (8 × 8), the luminance (59 cd/m2), and contrast level (80%) were kept constant for all the recordings in all the cases. The recording was done monocularly for the left and right eyes separately with the participant wearing corrective glasses if any during the test. If the cooperation of the participant or fixation stability was poor, the VEP recording was repeated after 5 min break. If the recorded signal was suboptimal, the VEP recording was repeated until a satisfactory recording was achieved.
Ethics consideration
The research protocol for the present study was submitted to the Institutional Ethics Committee, and we received ethical clearance before the commencement of the study. Informed consent was submitted by all participants before the investigation. All research and data collection protocols complied with the Declaration of Helsinki.
Statistical analysis
SPSS 23.0 Version, (IBM, USA) was used to enroll sociodemographic and clinical variables for both case (s) and control and to carry out descriptive and inferential statistics. One-way ANOVA was used to test significant differences between the six groups. Significant differences between each paired groups were then evaluated by post hoc Tukey's test.
RESULTS
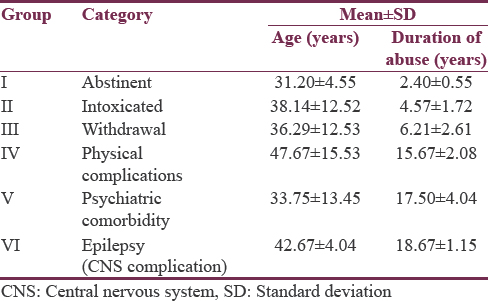
A total of 668 sample subjects were screened for obtaining 15 patients each in six subgroups of alcohol spectral severity for studying VEP abnormalities. The mean age of 90 cases of alcohol dependence was 37.71 ± 11.49 years while that of 180 healthy age- and sex-matched volunteers was 39.43 ± 10.67 years while their mean age did not differ significantly and both groups were comparable for age and gender as seen from Table 1. Among six different subgroups of alcohol use disorder/related complications, participants who had physical complications were found to be the oldest among six subgroups with mean age of 47.67 ± 15.53 years while those who have been abstinent for more than 6 months were the youngest (31.20 ± 4.55 years). Mean age of onset for psychiatric comorbidity was far earlier (33.75 ± 13.45 years) than both central nervous system (CNS)-induced complications (42.67 ± 4.04 years) and physical complications.
Tables 2 and 3 show quantitative analysis of VEP using one-way ANOVA for six subgroups so as to identify which of them (N70 latency, P100 latency, N155 latency, and P100 amplitude) might have significant impact on differentiating six subgroups of alcohol spectral severity. We found N70 latency getting significant and distinctive findings in the subgroups of physical complications and psychiatric comorbidities. However, P100 latencies were found to be significantly prolonged in alcohol intoxication, alcohol withdrawal, alcohol-induced physical, psychiatric and neurological complications; therefore, P100 latency delay could be a common, but indistinguishable pathway for alcohol-induced neural insults as observed in our study. When we further compared N155 latencies among these six subgroups, only those with hepatic encephalopathy (physical complications) were found to have highest delay and could be significantly differentiated from the other subgroups. Going a step further, P100 amplitude was significantly lower in patients of alcohol withdrawal and patients of hepatic encephalopathy and alcohol-induced epilepsy, providing us another matrix of association wherein dual associations could be formed. Thus, P100 latency delay and P100 amplitude reduction both are found to be significant only in the subgroup of alcohol-induced epilepsy which can be differentiated from the subgroup of intoxicated patients where P100 amplitude reduction is insignificant, and alcohol withdrawal patient can be differentiated from the patient of alcohol-induced epilepsy when N70 latency parameter has been used.
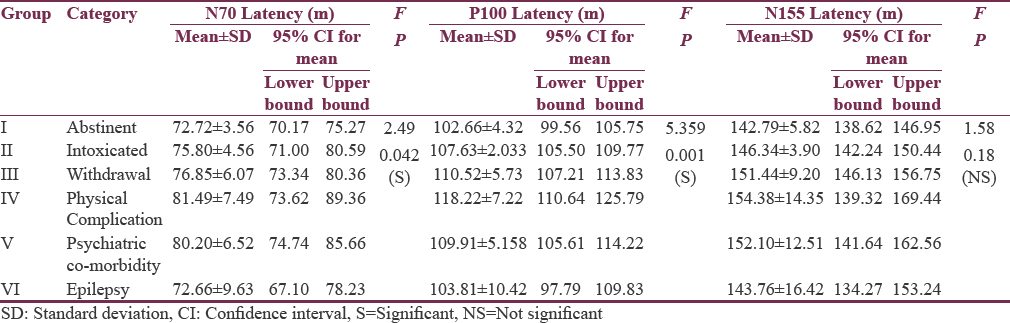
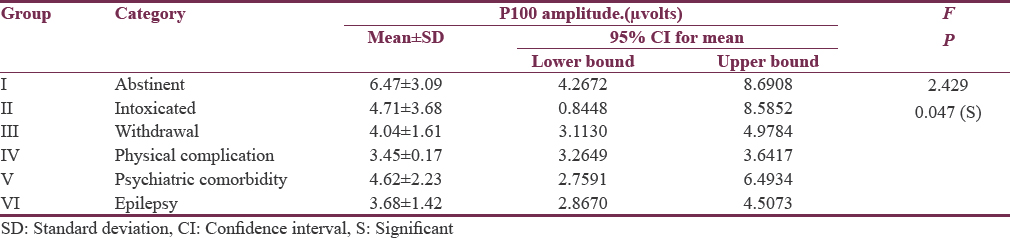
All these quantitative results are also depicted in Figure 1.
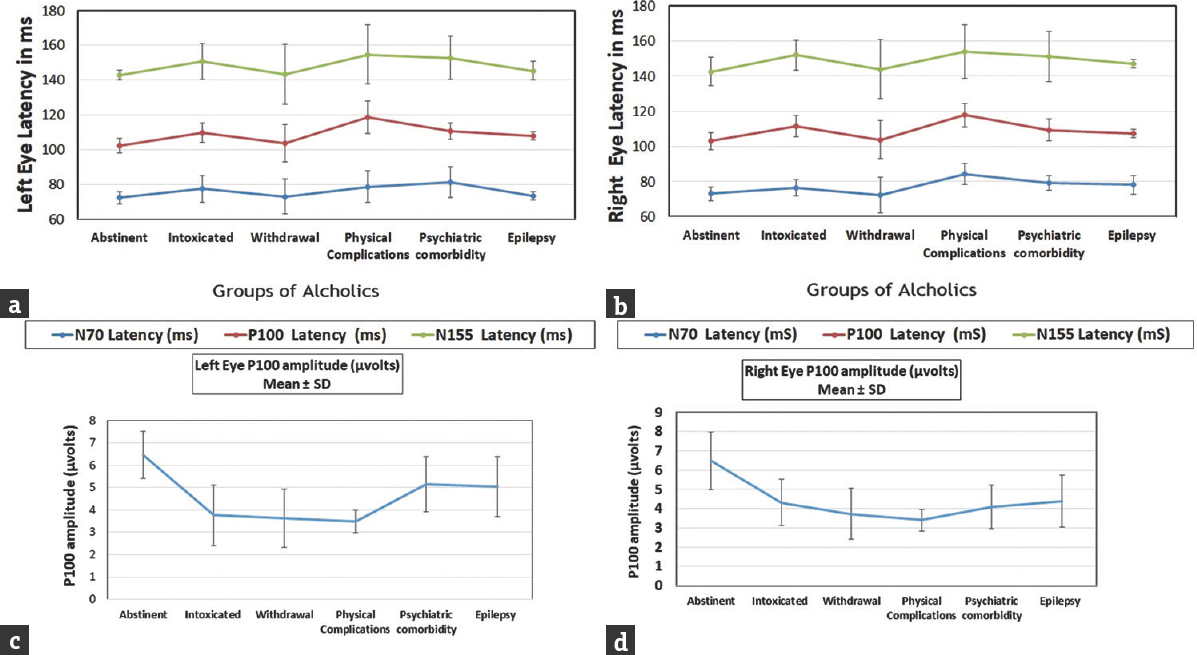
- Integration of quantitative results. (a) VEP Latencies of left eye, (b) VEP Latencies of right eye, (c) P100 amplitude in left eye, (d) P100 amplitude in right eye
Nevertheless, as shown in Table 4, not all patients necessarily resulted in such observations. The relative percentage of abnormal waveform morphology, latency delay, and amplitude reduction could be segregated with variable quantum of patients, for example, predominantly extended latencies (62.5%) with a statistically highly significant difference (P < 0.001) as compared to healthy controls were observed in cases of alcohol withdrawal syndrome while severe VEP abnormality as in both latency delay and amplitude reduction was found in 75% patients with psychiatric comorbidity and 66.67% patients with neurological complications. Interestingly, an explicit finding of prominent interocular differences was a feature present in 25% of patients with complications. Finally, abnormal waveform morphology, poorer reproducibility, and differentiation ability of the evoked complex which frequently had an atypical shape were obtained in 33.34%. A composite view of representative waveforms obtained in each group is illustrated in Figure 2.
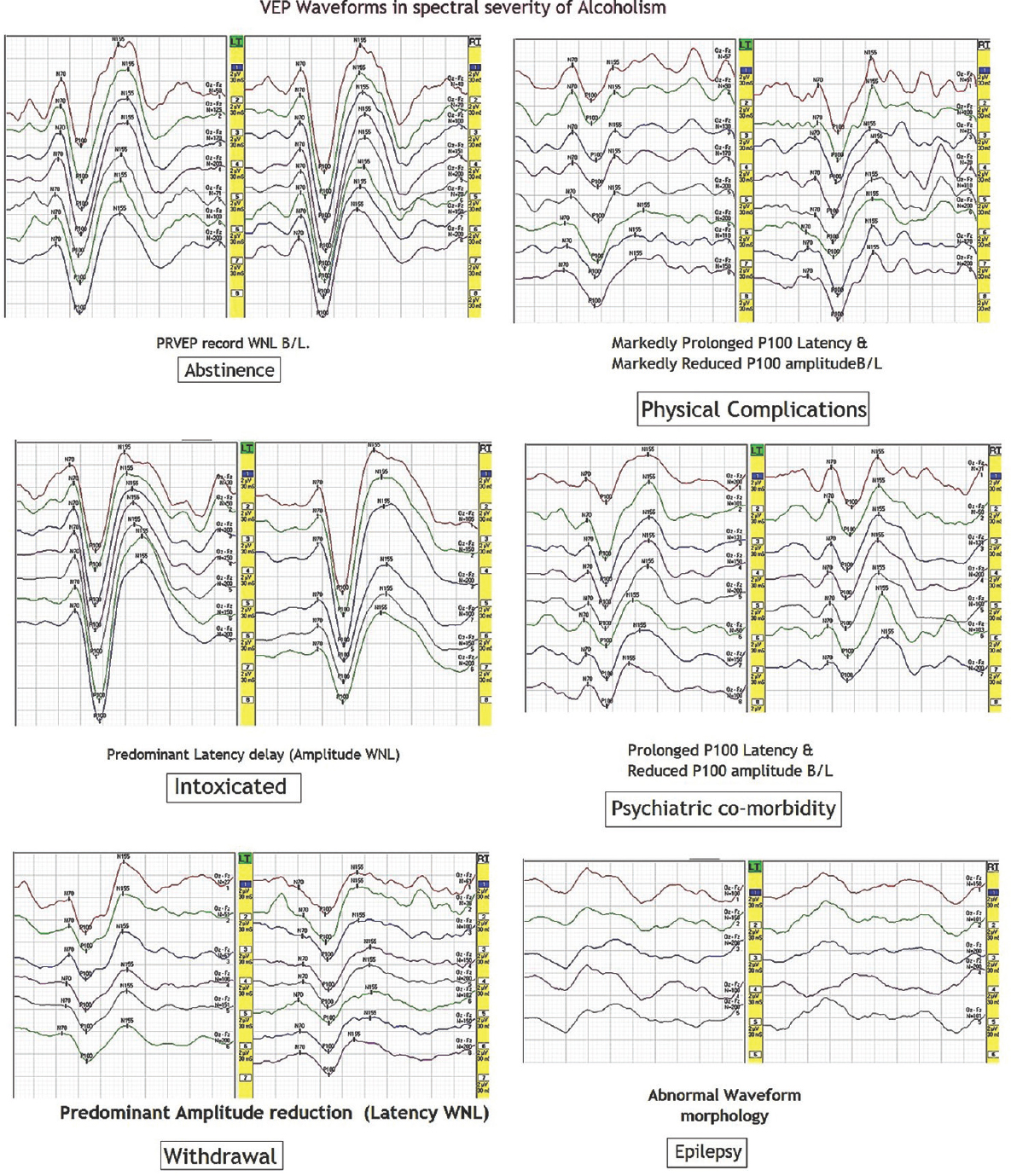
- Visual-evoked potential waveforms in the spectral severity of alcoholism
DISCUSSION
Alcohol-induced physical/neuropsychiatric complications are etiologically and phenotypically complex. It is evident that a chronic alcohol exposure typically leads to Vitamin B12 or folate deficiency, and over a time, these deficiencies cause accumulations of formic acid and its derivatives, which further inhibit(s) the electron transport chain and mitochondrial function, resulting in the disruption of Adenosine Triphosphate (ATP) production and ultimately impairing the ATP-dependent axonal transport system. Clinicians often need to detect early complications or ideally pick up vulnerable patients for whom such complications are likely to happen in the near future; however, unfortunately, there are hardly any biologically identifiable ways/markers to discern such disposing states. The acute effects of alcohol on the visual cortical/neural system have previously been evaluated with VEP;[67819202122] some of them validated VEP to help in differentiating from normal or alcoholic withdrawal patients, but none of them ever thought for spectral severity or set of specific emerging VEP patterns that might predict the endophenotypic state of the alcohol-induced neuropsychiatric complications. The N75, P100, and N135 components are found to be generated from the striate cortex (V1) or the extrastriate cortex. Alcohol-induced CNS suppression and nerve conduction delay have been reported to be mediated by gamma-aminobutyric acid (GABA), glycine, and adenosine.[23] GABA and glycine are the main inhibitory neurotransmitters in the CNS. Alcohol also increases the level of adenosine, which contributes to the sedative actions of alcohol. Furthermore, interactions of alcohol with myelin or the Ca-ATPase pump at the synapses also may explain these neurophysiological changes.[24] Thus, our study is the first one documented to evaluate and putatively search for optimal neurophysiological biomarkers for differentiating alcohol spectral severity by empirically identifying specific set of patterns obviously discernable (if any) on VEP in six divided set of subgroups of patients. The previous data from >20 studies[92526272829303132333435] [Table 5] showed few robust pointers for usefulness of VEPs in relation to alcohol use disorder (s) not only in in vivo animal experimentations but also in humans helping to differentiate subgroup of patients who had been abstinent for more than 3 months to those who are either intoxicated or having withdrawal. To our surprise, we noted couple of critical studies by Urban et al. and Nazliel et al., who reported that only 28% and 15% of alcoholics had abnormal findings on VEP, which is in contrast to most studies including ours with 75% patients with psychiatric comorbidity and 66.67% patients with neurological complications. Certainly, this is very unique way of neurophysiological differentiation on spectral severity of alcoholism as observed and conceptually derived for the first time ever in the literature [Table 4] from our study. Nevertheless, such relative percentage of abnormal waveform morphology, latency delay, and amplitude reductions in VEP as biomarker clearly holds us back in reaching to unified consolidative neurobiological markers until our understanding and technological advances get widen in the future. An ultimate and implicit goal of the work described in this article is to develop more effective prevention techniques. Increased understanding of the biological mechanisms and genetic impact associated with a specific type of increased vulnerability to alcoholism could enhance prevention efforts in several ways. For instance, PRVEP abnormalities obtained in asymptomatic chronic alcoholics of our study suggest that they may be useful in the detection of early changes and in following the progress of cases with the chronic addiction. This study adds further evidence and emphasis to the sparse literature of usefulness of VEP in a wide spectrum of alcohol abuse seen in young adults. This also helps to establish the importance of a visual electrophysiological evaluation as a valuable adjunct to detailed psychiatric assessment of alcoholics and provides a decent recommendation for VEP to be a part of their routine examination as it might be a useful marker which may help to clearly classify the spectral severity of alcoholism and help in early rehabilitation of addicts.

Given the multiplicity of experimentally driven and empirical clinical findings of VEP in spectrum of alcoholism, we could elucidate the ingredient of progressively more profound and consistent observable patterns of association that might potentially differentiate the kind of variety of alcohol-related spectra. The importance of a neurophysiological marker's (VEP) precision, accuracy, sensitivity, and specificity cannot be overemphasized. Although it is unlikely that researchers will find a single marker to satisfy all clinical needs, they may eventually develop combinations of markers for specific clinical purposes, from unselective screening (i.e., drinking versus not drinking) to confirming a suspicion of alcohol induced. The conceptual overview espoused here is ripe for both further preclinical experimental testing and exploratory therapeutic interventions. Should this prove to be true, our findings we believe would contribute to a stronger basis for clinical care and a more objective assessment of alcohol-related complications or help in identifying that might relapse or would have neuropsychiatric complications in the future and such phenomena deserve explorations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiologic catchment area (ECA) study. JAMA. 1990;264:2511-8.
- [Google Scholar]
- The effect of alcohol and substance abuse on the course of bipolar affective disorder. J Affect Disord. 1996;37:43-9.
- [Google Scholar]
- Evaluation of the central effects of alcohol and caffeine interaction. Br J Clin Pharmacol. 1995;40:393-400.
- [Google Scholar]
- Simple reaction time event-related potentials: Effects of alcohol and sleep deprivation. Alcohol Clin Exp Res. 1993;17:771-7.
- [Google Scholar]
- Visual evoked response and alcohol intoxication. Acta Ophthalmol (Copenh). 1984;62:651-7.
- [Google Scholar]
- Effects of low alcohol consumption on visual evoked potential, visual field and visual contrast sensitivity. Acta Ophthalmol Scand. 1999;77:23-6.
- [Google Scholar]
- Event-related potentials during visual target detection in treatment-naïve active alcoholics. Alcohol Clin Exp Res. 2011;35:1171-9.
- [Google Scholar]
- Alcohol-related context modulates performance of social drinkers in a visual Go/No-Go task: A preliminary assessment of event-related potentials. PLoS One. 2012;7:e37466.
- [Google Scholar]
- Reduced processing of alcohol cues predicts abstinence in recently detoxified alcoholic patients in a three-month follow up period: An ERP study. Behav Brain Res. 2015;282:84-94.
- [Google Scholar]
- Occipital event-related potentials to addiction-related stimuli in detoxified patients with alcohol dependence, and their association with three-month relapse. BMC Psychiatry. 2016;16:74.
- [Google Scholar]
- Relationship between alcohol dependence, escape drinking, and early neural attention to alcohol-related cues. Psychopharmacology (Berl). 2014;231:2031-40.
- [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (4th ed). Washington, D.C: American Psychiatric Publishing, Arlington, VA; 2000.
- [Google Scholar]
- Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1996.
- [Google Scholar]
- Recommended standards for electroretinograms and visual evoked potentials. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1993;87:421-36.
- [Google Scholar]
- ISCEV standard for clinical visual evoked potentials (2009 update) Doc Ophthalmol. 2010;120:111-9.
- [Google Scholar]
- Visual evoked potentials in chronic alcoholics. Cesk Neurol Neurochir. 1989;52:271-6.
- [Google Scholar]
- Dose dependent effects of alcohol on visual evoked potentials. Psychopharmacology (Berl). 1993;112:383-8.
- [Google Scholar]
- Alcohol and neurotransmitter interactions. Alcohol Health Res World. 1997;21:144-8.
- [Google Scholar]
- The interaction of ethanol with reconstituted synaptosomal plasma membrane Ca2+ -ATPase. Biochim Biophys Acta. 2004;1665:75-80.
- [Google Scholar]
- Alcoholism: Averaged visual evoked response amplitude-intensity slope and symmetry in withdrawal. Biol Psychiatry. 1976;11:435-43.
- [Google Scholar]
- Simultaneous recording of visually evoked potentials (VEP) and registration of simple visual reaction times (RT) in the maximum range of ethanol influence (author's transl) Arzneimittelforschung. 1976;26:1125-6.
- [Google Scholar]
- Ethyl alcohol effect on the visual evoked potential. Acta Physiol Pol. 1981;32:93-8.
- [Google Scholar]
- Ethanol and menstrual cycle interactions in the visual evoked response. Electroencephalogr Clin Neurophysiol. 1981;52:28-35.
- [Google Scholar]
- Effects of age and alcohol abuse on pattern reversal visual evoked potentials. Clin Electroencephalogr. 1984;15:102-9.
- [Google Scholar]
- EEG, visually evoked and event related potentials in young abstinent alcoholics. Alcohol. 1987;4:241-8.
- [Google Scholar]
- Evaluation of peripheral nerve conduction and central visual conduction in chronic alcoholics and in chronic alcoholics after prolonged abstention. Ital J Neurol Sci. 1988;9:583-92.
- [Google Scholar]
- Pattern reversal visual evoked potential among men at risk for alcoholism. Acta Psychiatr Scand. 1988;78:276-82.
- [Google Scholar]
- An effect of alcohol on the distribution of spatial attention. J Stud Alcohol. 1996;57:260-6.
- [Google Scholar]
- The effects of alcohol on visual evoked potential and multifocal electroretinography. J Korean Med Sci. 2016;31:783-9.
- [Google Scholar]






