Translate this page into:
Hyperbaric oxygen therapy improves recovery at acute motor axonal neuropathy case
*Corresponding author: Ni Komang Sri Dewi Untari, Department of Neurology, Dr. Ramelan Navy Hospital, Surabaya, Indonesia. komang_neuro@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Untari NK, Kusumastuti K, Suryokusumo G, Sudiana IK. Hyperbaric oxygen therapy improves recovery at acute motor axonal neuropathy case. J Neurosci Rural Pract 2023;14:145-8.
Abstract
Acute motor axonal neuropathy (AMAN) is a rare immune-mediated disorder characterized by acute flaccid paralysis with elevated levels of GM1 antibodies. It is also known as a subtype of the Guillain-Barre syndrome (GBS) and develops since antigen s serve as antibodies in the spinal cord. We report a case diagnosed as AMAN with symptoms of ascending limb symmetrical weakness. A neurological examination revealed a flaccid paralysis with multiple cranial nerve palsies. Electromyography showed an axonal type of GBS. The patient refused bone marrow fluid aspiration. Intravenous immunoglobulin was administered at the high care unit. Unfortunately, despite the standard therapy, an optimal recovery was not obtained. Hyperbaric oxygen (HBO) therapy has been known to be common in illnesses and some clinical diseases. Although it has not been indicated for peripheral neuropathy, a remarkable recovery was soon visible in the HBO-treated AMAN case. The HBO mechanisms involved here are anti-inflammation and immunomodulation.
Keywords
Guillain-Barre syndrome
Hyperbaric oxygen
Acute motor axonal neuropathy
INTRODUCTION
Acute motor axonal neuropathy (AMAN) is an axonal subtype of the Guillain-Barre syndrome (GBS) and was first described in 1986 by Feasby et al. Clinical symptoms of AMAN are characterized by acute limb weakness, often accompanied by respiratory failure and sometimes by cranial nerve disorders and vegetative involvement.[1] Existing evidence suggests the presence of peripheral nerve-dependent autoantibodies, followed by complement deposition that results in neuronal damage in both the acute inflammatory demyelinating polyradiculoneuropathy (AIDP) and AMAN types.
Most cases of mild GBS respond to conventional treatment with high doses of intravenous immunoglobulin (IVIg).[2] In the patient, the response to therapy was poor and the post-therapeutic residue was visible. He remained severely disabled (second discharge) when he was discharged. In 2016, we treated a woman diagnosed with AMAN who recovered within 20 days of receiving the hyperbaric oxygen (HBO) therapy.[3] With the same procedure as that of the above case of patient, this patient also underwent HBO therapy for 20 times. In this study, we report a case of AMAN with symptoms of acute limb weakness, who recovered more quickly with the HBO therapy.
CASE REPORT
A 49-year-old male patient presented to the emergency room with a history of acute lower limb weakness. This weakness occurred 1 day before admission to the hospital. The weakness moved upward, with weakness in the right and left arms and difficulty breathing. His condition got worse the day before admission with the development of progressive bilateral paws, facial weakness, slurred speech, and dysphagia. He had a history of diarrhea approximately 3 weeks earlier. The patient had no medical history of diabetes, high blood pressure, or heart attacks. The family history revealed that no one had the same symptoms and signs.
An oxygen mask of 10 L/min and a nasogastric tube were given to the patient. Despite the 5-day IVIg therapy, there was minimal improvement. The patient was more difficult to breathe and had difficulty swallowing. On the 3rd day, the patient was admitted to the ICU and placed next to the ventilator. On the 5th day at the ICU, the patient was stable without using a ventilator, only NPA, routine nebulization, and suction. On the 9th day, he underwent the therapy in the Neurology ward. His family had practiced how to treat the patient, from tilting, patting the chest to stimulating sputum, feeding and drinking through a nasogastric tube, and ensuring that the patient was not dehydrated by monitoring the urine bag. Since the patient’s condition was stagnant for 2 days, HBO therapy was considered [Figure 1].
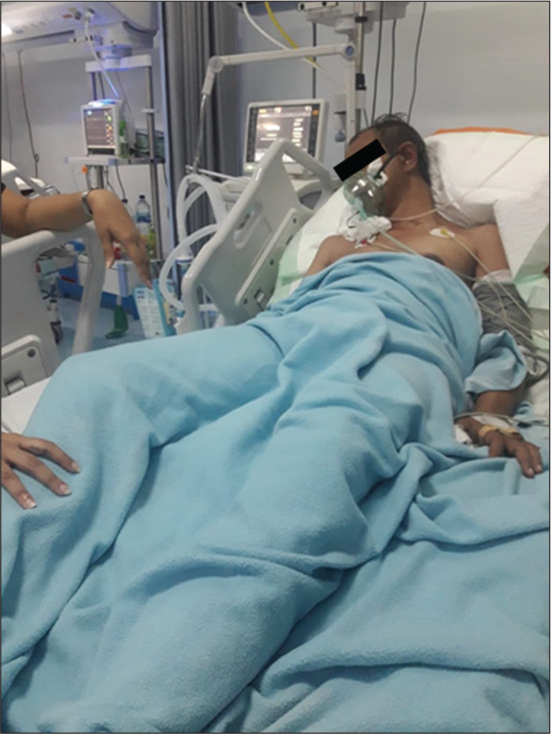
- Man, 49 years old, diagnosed as an axonal type of GBS, with having Intravenous Immunoglobulin at Intensive Care Unit. Note the dropping hand, and inability to swallow and breathe normally.
The patient laid on a gurney when admitted to the hyperbaric unit. The systemic examination showed stable vital signs and generalized muscle tenderness. Neurological examination revealed a normal mental status. Several abnormalities were found on the cranial nerves. Bilateral facial muscle weakness led to mask-like facial expressions. His speech was slurred with palatal weakness. Weak swallowing indicated bulbar involvement. Sensory examination revealed hypoesthesia of both extremities. The deep tendon reflexes of the upper and lower extremities were reduced (+ 1) and the proximal and distal muscle strength of the upper extremities was 3/3/3/2|2/2/2/2 and the lower leg was 2/1/1/1|1/1/1/2. He could only raise his hand minimally. The laboratory results were as follows: WBC: 15,000; lymphocytes: 70%; PMN: 30%; Hb: 13; and CRP: +3. The patient refused the lumbar puncture procedure. Electromyographic examination was diagnostic of an axonal type of GBS.
At a hyperbaric chamber, the patient inhaled 100% oxygen through a mask for three cycles of approximately 30 min at a depth of 14 m. The session last for up to 2 h and was conducted daily for 10 consecutive days. After completing the first cycle (10 times of HBO session), the patient rested for 2 days. On day 13, the patient continued on the HBO sessions again for another 10 days [Figure 2].
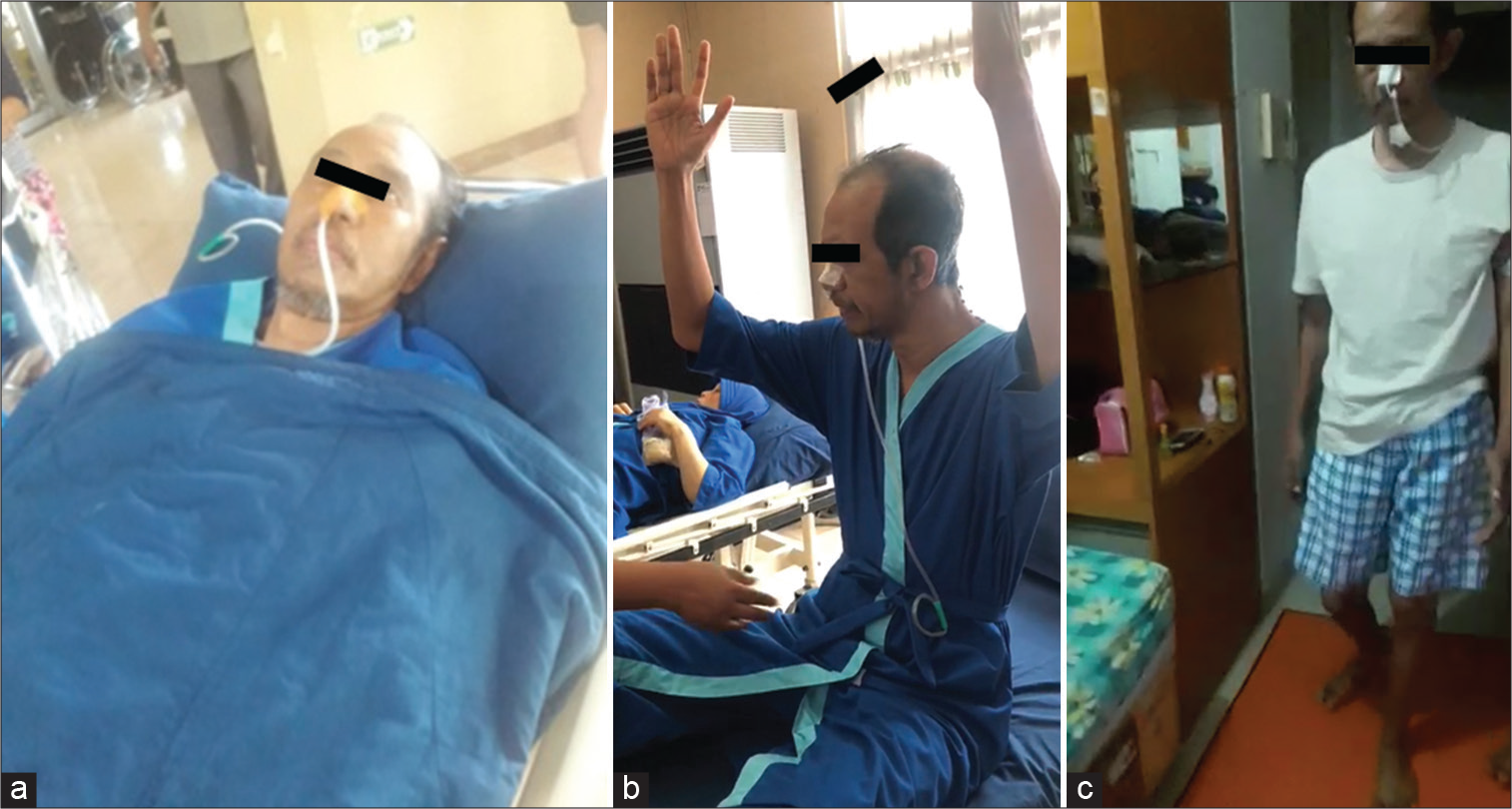
- The first patient when undergone hyperbaric therapy (a), 2nd day (b), 10th day (c), 20th day.
On the 1st day of the HBO session, the patient was able to sip water using a spoon. On day 2, the patient was able to suck water using a straw. Finger movements were more pronounced on the 5th day, while the foot movements occurred on the 7th day. On day 10, the patient was able to raise his arms and bend his legs. He was able to sit by himself on the bed on the 13th day. The patient was found sitting with his legs dangling on the bedside on the 13th day. On day 17, the patient tried to stand with the help of a physiotherapist, despite the inability to walk. On day 20, he was able to limp a maximum of five steps without using a walking aid.
DISCUSSION
AMAN is an axonal subtype of the GBS which has a more severe course than classic GBS. Paralysis or weakness is the residual symptom experienced by patients.[1] GBS is an autoimmune neuropathy with a high rate of mortality. Gangliosides (self-enriched glycan-carrying molecules in neural tissue) are considered to be major antigenic targets for antibodies that mediate this deactivation process.[4] GBS, the prototype post-infectious dysimmune neuropathy, has for some time been described as a AIDP with predominant T-cell-mediated immunopathogenesis.[1,5] About 5–10% of patients recovering from GBS may relapse within 3 days– 3 weeks despite the therapy.[6] AMAN is common among young people in Japan and China and during the dry season. AMAN is associated with the previous infection with Campylobacter jejuni.[7] The patient in this case report belonged to the adult category, who had the disorder in the dry season and had a history of gastrointestinal symptoms.
T-cell costimulation plays a central role in autoimmunity. Infection may enhance autoimmunity by inducing costimulatory activity in antigen-presenting cells, which are required to induce the expansion of antigen-reactive T cells, migration of T cells to inflammatory sites, and production of inflammatory mediators.[8] Molecular mimicry between microorganisms and gangliosides may induce the development of anti-GM1 or anti-GD1a IgG antibodies in patients with axonal GBS. Pathogenic autoantibodies bind to GM1 and activate complement at the nodes of Ranvier in peripheral nerves, which lead to the formation of a membrane attack complex at the nodal axolemma, resulting in muscle weakness.[9]
Only a small number of individuals infected with C. jejuni develop GBS. Bacterial risk factors are genes or gene polymorphisms located at lipo-oligosaccharide (LOS) gene cluster, highlighting the important role of LOS in the pathogenesis of Campylobacter-associated GBS. However, family studies showed that although many individuals can be infected with the same type of Campylobacter, not all family members develop GBS. These observations also revealed that, in addition to bacterial characteristics, host-dependent factors also play a role in the development of post-Campylobacter neuropathy.[10]
As a traditional first-line therapy, high-dose IVIg therapy is effective in several autoimmune disorders by regulating autoreactive T/B-cell function. In general, the therapeutic effect of IVIg appears to be through direct competition with autoantibodies by neutralizing autoantibodies by anti-idiotypic antibodies and by inhibiting complement deposition.[9] Che et al. reported no significant difference in the post- and pre-therapeutic percentage of ICOS+ and programmed death-1 (PD-1) + cells between Tfh17 and plasmablast count in severe AMAN patients. This renders IVIg ineffective in severe AMAN, due to poor modulation of Tfh-B cell interactions.[2]
With regard to the ineffectiveness of IVIg in AMAN, the current research focuses on seeking adjuvant therapies including the disease-triggering inflammatory and immunologic mechanisms. HBO is a method of treating patients by entering them into a high-pressure room. Patients wear a mask and inhale 100% oxygen and are given more than 1 absolute atmospheric pressure (ATA) for a certain period of time.[11] Most hyperbaric applications are derived from the principles of the laws of physics. First, Boyle’s law states that the volume of a gas is inversely proportional to the pressure. Second, Dalton’s law states that the pressure of a gas mixture is the sum of the partial pressures of the constituent gases. Finally, Henry’s law states that pressure is proportional to the number of particles dissolved in it.[12] Delivery of 100% oxygen under high pressure causes a systemic increase in blood oxygen concentration, whereas high pressure leads to a significant increase in oxygen transfer from the blood to all body tissues.[13]
Hypoxia, which can be the first step in axonal degeneration , is the reason behind the use of HBO. HBO is often used in the treatment of ischemic wounds, such as acute trauma, refractory wounds, tissue flaps, and transplants with poor blood circulation and radiation-induced bone death.[14] HBO is reported to improve neurologic recovery after bone marrow injury by correcting mitochondrial dysfunction in the motor cortex and bone marrow, stop the spread of bleeding, reverse hypoxia, and reduce edema.[15,16] In experimental animals, HBO therapy can reduce axonal degeneration by triggering GSH activity, increasing IL-1β levels, and restoring tissue and motor status. In conclusion, HBO has a protective effect on spinal cord and sciatic nerve degeneration in the AMAN rabbit model.[17] In addition, HBO affects the M1 to M2 phenotypic shift by decreasing the expression of HIF-1α while increasing the production of IL-10, thereby reducing arthritis in collagen-induced arthritis.[18] We expect HBO to have benefit in peripheral neuropathy as well.
CONCLUSION
AMAN is a peripheral nerve disorder with clinical manifestations of ascending limb weakness and capable of progressing to diaphragmatic and bulbar paralysis. Autoimmune mechanisms which are a continuation of the inflammatory process are involved in the course of AMAN disease. HBO therapy, which has anti-inflammatory effects and regulatory T-lymphocyte recruitment, is beneficial in autoimmune inflammatory neuropathies.
With regard to the ineffectiveness of IVIG in patients with AMAN, the outcomes of HBO-treated AMAN patients appear to be remarkable. This supports the point that regulatory T lymphocyte recruitment through HBO may have an additional therapeutic effect on autoimmune inflammatory neuropathies.
Acknowledgment
We would like to thank for providing hyperbaric chamber support.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- An acute axonal form of Gguillain-Barrée polyneuropathy. Brain. 1986;109:1115-26.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of an acute motor and sensory axonal neuropathy with propionate in a 33-year-old male. Ther Adv Neurol Disord. 2018;11:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of hyperbaric oxygen treatment on axon degeneration after acute motor axonal neuropathy. Autoimmune Dis. 2021;2021:6627779.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating memory T follicular helper subsets, Tfh2 and Tfh17, participate in the pathogenesis of guillain-barré syndrome. Sci Rep. 2016;6:20963.
- [CrossRef] [PubMed] [Google Scholar]
- Acute motor axonal neuropathy improvement 20 days after hyperbaric oxygen therapy. Int Med Case Rep J. 2021;14:151-5.
- [CrossRef] [PubMed] [Google Scholar]
- Experimental guillain-barre syndrome induced by immunization with gangliosides: Keyhole limpet hemocyanin is required for disease triggering. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1473-8.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging markers of clinical progression in chronic inflammatory demyelinating polyradiculoneuropathy. Ther Adv Neurol Disord. 2019;12:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding guillain-barré syndrome. J Am Acad Physician Assist. 2015;28:19-22.
- [CrossRef] [PubMed] [Google Scholar]
- A new animal model of spontaneous autoimmune peripheral polyneuropathy: Implications for guillain-barré syndrome. Acta Neuropathol Commun. 2014;2:1-14.
- [CrossRef] [PubMed] [Google Scholar]
- Polyclonal IgM and IgA block in vitro complement deposition mediated by anti-ganglioside antibodies in autoimmune neuropathies. Int Immunopharmacol. 2016;40:11-5.
- [CrossRef] [PubMed] [Google Scholar]
- Host factors determine anti-GM1 response following oral challenge of chickens with guillain-barré syndrome derived campylobacter jejuni strain GB11. PLoS One. 2010;5:e9820.
- [CrossRef] [PubMed] [Google Scholar]
- Physical, physiological and biochemical aspects of hyperbaric oxygenation In: Textbook of Hyperbaric Oxygen. London: Springer; 2017.
- [CrossRef] [Google Scholar]
- Physics and physiology In: Edmonds C, Bennett M, Lippmann J, Mitchell SJ, eds. Diving and Subaquatic Medicine. Boca Raton: CRC Press; 2016.
- [Google Scholar]
- Immunosuppressants: Neuroprotection and promoting neurological recovery following peripheral nerve and spinal cord lesions. Exp Neurol. 2005;195:7-15.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of hyperbaric oxygen therapy on nerve regeneration in early diabetes. Microsurgery. 2004;24:255-61.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and outcomes of guillain-barré syndrome among pediatrics in Saudi Arabia: A 10-year retrospective study. Neuropsychiatr Dis Treat. 2019;15:627-35.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of hyperbaric oxygen on t helper 17/regulatory t polarization in antigen and collagen-induced arthritis: Hypoxia-inducible factor-1α as a target. Oman Med J. 2020;35:e90.
- [CrossRef] [PubMed] [Google Scholar]







