Translate this page into:
High Fructose Negatively Impacts Proliferation of NSC-34 Motor Neuron Cell Line
Jamuna R. Subramaniam Centre for Preclinical and Translational Medical Research, Sri Ramachandra Institute for Higher Education and Research Chennai 600116, Tamil Nadu India jamuna17@sriramachandra.edu.in drjamunarsubramaniam@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objectives The main aim of this study is to identify the deleterious effects of indiscriminately consumed high fructose on motor neurons that are critically affected in many neurological conditions causing movement disorders including paralysis.
Materials and Methods Neuroblastoma x mouse spinal cord motor neuron cell line (NSC-34) motor neuron cell lines were treated with high fructose and oxygen supplementation (18.8%) and assayed for cell proliferation/death, reactive oxygen species (ROS) generation, and oxidative stress response induction
Statistical Analysis Mean and standard deviation, significance with and without high fructose (F)-5%, were estimated by t-tests using GraphPad Prism ver. 8.2.1
Results F-5% along with O2 (18.8%) annihilates the cells (∼85%) by day10 and inhibits cell division as observed by the presence of multinucleated cells. Unexpectedly, 1 to 2% of cells that survived, differentiated and displayed progressive neurite extension. Though not healthy, they were viable up to 80 days. F-5% increased ROS levels (∼34%) not accompanied by concomitant enhanced expression of oxidative stress response regulator, the transcription factor, nrf-2, or downstream effector, sod-1.
Conclusion High fructose is extremely harmful to NSC-34 motor neuron cell line.
Keywords
fructose
NSC-34
motor neurons
cell proliferation
ROS
cell death
Introduction
Fructose, commercially inexpensive and highly addictive,1 commonly used in the form of refined sugar, sucrose, or high fructose corn syrup in confectioneries, baked/processed food, and sports drinks, is associated with disruption of metabolism. High fructose consumption with a sedentary lifestyle2 leads to various diseases like metabolic syndrome, hypertension, diabetes mellitus, obesity,3 and nonalcoholic fatty liver.4. Off-late, fructose is implicated in various neurological conditions, neuroinflammation,5 and neurodegenerative diseases (ND)6 as it crosses the blood–brain barrier in small quantities. In the brain, surplus glucose is converted to fructose through the sorbitol pathway and metabolized.7 Sports people are known to consume energy drinks enriched with glucose and fructose to replenish their energy rapidly. Sports personnel have a higher incidence of the ND, amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig disease, characterized by motor neuron (MN) degeneration leading to progressive paralysis and eventually death.8 Recent findings suggest that fructose metabolism is impaired in astrocytes9 of ALS patients having the C9orf72 repeat expansion.
Fructose is also known to generate extremely harmful reactive oxygen species (ROS), superoxide—O2 · and hydroxyl-OH, in hepatocytes.10 11 An established regulator of the oxidative stress defense mechanism, the nuclear factor erythroid-2 related factor (nrf-2), induces the expression of antioxidant response element-dependent genes that inactivate ROS and maintain redox homeostasis of the cells.12 A downstream effector of nrf-2, Cu/Zn superoxide dismutase I (sod-1), which dismutates O2 §− and converts it to H2O2 in the cytoplasm and mitochondrial intermembrane space, if mutated is known to cause ALS.13 14 Since fructose plays a major role in energy metabolism and ROS generation, its effects on oxidative stress response need to be better understood.
In this report, we address the effects of high fructose on cell proliferation, induction of morphological changes in the cells, and its role in ROS generation and antioxidant defense in the MNs.
Materials and Methods
Cell Culture and Assays
Neuroblastoma x mouse spinal cord motor neuron cell line (NSC-34)15 was maintained using standard protocols as reported earlier.6 16 17 The cells were maintained at 37°C with 5% CO2 and 18.8% O2 in the Forma series II CO2 incubator (Thermo Fischer Scientific, Waltham, Massachusetts, United States) and subcultured every 3 to 4 days. All the reagents were from Gibco (Life Technologies, Gaithersburg, Maryland, United States) unless otherwise mentioned. Fructose at a final concentration of 5% (277mM) was added to the cells seeded in T25 flasks (2 × 105 cells) in Dulbecco's modified Eagle's medium (DMEM) complete medium and this was maintained at 37°C with 5% CO2 and 18.8% O2 for the long-term assay. Cell viability was determined using thiazolyl blue tetrazolium bromide (MTT) assay as reported earlier.18 Further, 5% fructose treated cells were stained with acridine orange (50 µg/mL, 30 minutes, 37°C) that emits green fluorescence when bound to double-stranded DNA.19 After phosphate buffered saline washes, the cells were subjected to fluorescence microscopy. The cellular ROS levels were measured by using 2¢,7¢-dichlorofluorescin diacetate (DCF-DA) (Sigma Aldrich, Bengaluru, Karnataka, India).20 Expression of oxidative response pathway genes, Nrf-2 and Sod-1, was performed by reverse transcription polymerase chain reaction (RT-PCR). The cells were analyzed for nuclear staining in Nikon Eclipse-Ti fluorescence microscope, using fluorescein isothiocyanate filter. The nucleus was viewed with 10x and 40x objectives. The same microscope was utilized for viewing and capturing the phase-contrast images. The images were obtained with a CCD camera and Q-imaging software.
Statistics
Mean and standard deviation were calculated, and significance was estimated by t-tests using GraphPad Prism ver. 8.2.1 (GraphPad Software, La Jolla, California, United States).
Results
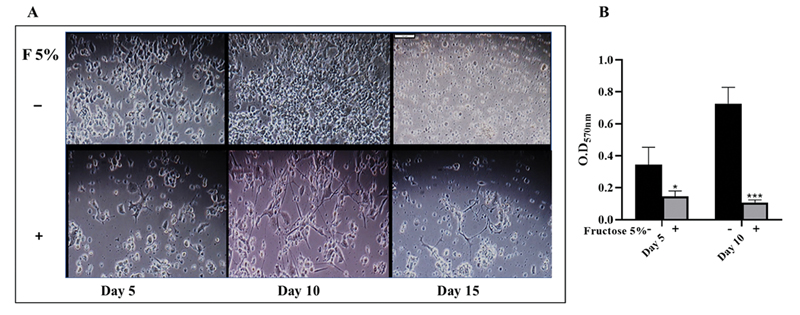
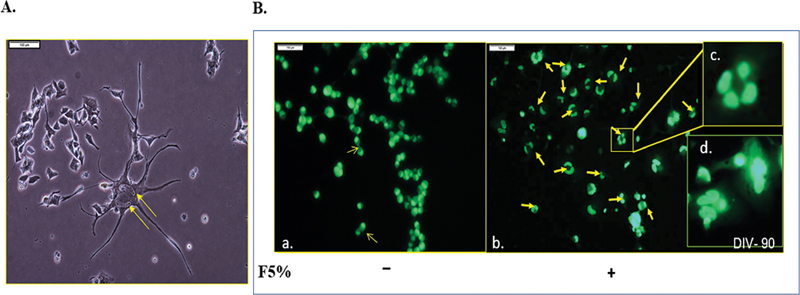
Treatment of the NSC-34 cell line with high fructose (5%) abrogated cellular proliferation as early as day 5 (Fig. 1A). This deteriorated further on day 10 and 15 (Fig. 1A). On day 5, cell death was 53%, determined by MTT assay (Fig. 1B) that became worse ∼85% by day 10 (day 5*–p = 0.017; day 10***– p = 0.0007) compared with control. To identity how high fructose inhibits NSC-34 proliferation, they were stained with acridine orange at day 20. This revealed multinucleated cells (Fig. 2). Although nuclear division was clearly apparent, cell division was obstructed. While control cells showed obvious cell division (Fig. 2B, a), it was almost negligible in F5% treated cells (Fig. 2B, b-d), a clear indication of cell division arrest at cytokinesis, thereby inhibition of cell proliferation leading to multinucleated cells. One possibility is the inefficient synthesis of the cellular components needed due to reduced or lack of ATP production21 upon F5% treatment.
-
Fig. 1 High fructose inhibits cell proliferation. (A) Deterred cell density with 5% fructose treatment as early as day 5 (Scale: 1 cm = 100 µm) (B) Significant cell death confirmed by thiazolyl blue tetrazolium bromide assay performed at day 5 and 10 (day 5*–p = 0.0275; day 10***–p < 0.0001).
Fig. 1 High fructose inhibits cell proliferation. (A) Deterred cell density with 5% fructose treatment as early as day 5 (Scale: 1 cm = 100 µm) (B) Significant cell death confirmed by thiazolyl blue tetrazolium bromide assay performed at day 5 and 10 (day 5*–p = 0.0275; day 10***–p < 0.0001).
-
Fig. 2 Multinucleation observed in most cells exposed to high fructose. (A) 5% Fructose inhibited cell division at cytokinesis. (B) a. Upon acridine orange staining, control cells show normal cytokinesis each divided cell with a single nucleus. b. 5% fructose led to binucleated, trinucleated, and polynucleated cells. c. Four nuclei (magnified inset) (Scale: 1cm = 100 µm). Nuclei are indicated with yellow arrows.
Fig. 2 Multinucleation observed in most cells exposed to high fructose. (A) 5% Fructose inhibited cell division at cytokinesis. (B) a. Upon acridine orange staining, control cells show normal cytokinesis each divided cell with a single nucleus. b. 5% fructose led to binucleated, trinucleated, and polynucleated cells. c. Four nuclei (magnified inset) (Scale: 1cm = 100 µm). Nuclei are indicated with yellow arrows.
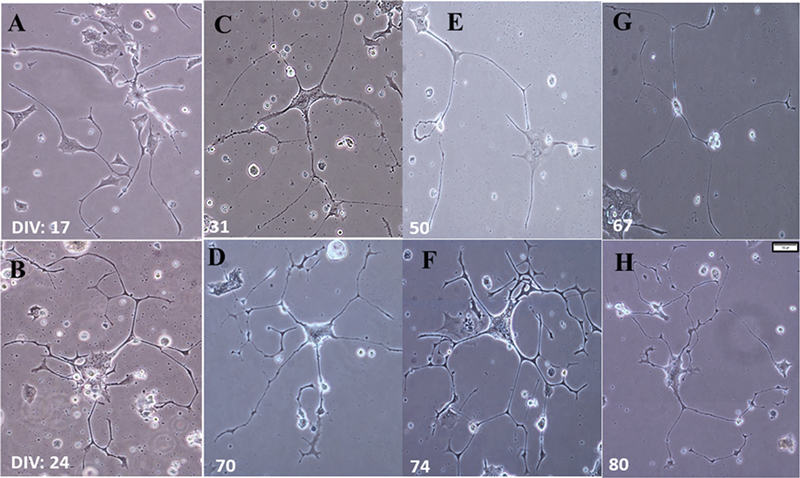
Contrary to the above-mentioned adverse effects, around 1 to 2% of cells exhibited clear-cut differentiation as observed by the presence of extensive neurites (Fig. 3). Of this populace, ∼0.5% survived up to day 80 when maintained with a medium change every 5 days and oxygen 18.8% (Fig. 3). This oxygen supplementation was essential for the long-term maintenance.
-
Fig. 3 Fructose effects—Differentiation and long-term survival in vitro (A–B). Fructose 5% treatment induced neuronal differentiation and facilitated neurite formation. (C–H) Differentiated cells at varied DIV that survived from 31 to 80 days. (DIV—No. of days in vitro in culture) (Scale: 1cm = 100µm).
Fig. 3 Fructose effects—Differentiation and long-term survival in vitro (A–B). Fructose 5% treatment induced neuronal differentiation and facilitated neurite formation. (C–H) Differentiated cells at varied DIV that survived from 31 to 80 days. (DIV—No. of days in vitro in culture) (Scale: 1cm = 100µm).
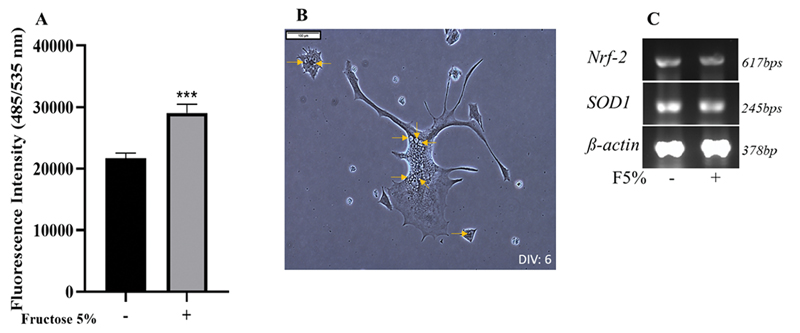
Fructose is known to increase ROS levels in various cell types.10 11 To identify fructose mediated ROS generation and its impact on NSC-34 cells, ROS levels were measured using the fluorescent ROS indicator, DCF-DA. F5% significantly increased DCF-DA fluorescence intensity by 34% upon 48 hours exposure (Fig. 4A), showing a significant increase in ROS levels (***–p = 0.0002). The neuronal cell body also exhibited an extensive number of vacuoles (Fig. 4B). This vacuolation is indicative of ROS mediated cellular stress and toxicity.22
-
Fig. 4 Neuronal reactive oxygen species (ROS) increases after high fructose treatment. (A) 2¢,7¢-dichlorofluorescin diacetate—fluorescence intensity measured ROS increased significantly (∼50%) in neurons exposed to high fructose for 48 hours (***–p = 0.0002) (B) Vacuoles (yellow arrows) in the cells exposed to high fructose (scale: 1cm = 100µm) (C) Expression of nrf-2 and sod-1 in neuroblastoma x mouse spinal cord motor neuron cell line cells by reverse-transcription-polymerase chain reaction. (-) – control; (+) – F5% treated. The levels are not altered with F5% exposure.
Fig. 4 Neuronal reactive oxygen species (ROS) increases after high fructose treatment. (A) 2¢,7¢-dichlorofluorescin diacetate—fluorescence intensity measured ROS increased significantly (∼50%) in neurons exposed to high fructose for 48 hours (***–p = 0.0002) (B) Vacuoles (yellow arrows) in the cells exposed to high fructose (scale: 1cm = 100µm) (C) Expression of nrf-2 and sod-1 in neuroblastoma x mouse spinal cord motor neuron cell line cells by reverse-transcription-polymerase chain reaction. (-) – control; (+) – F5% treated. The levels are not altered with F5% exposure.
Generally, ROS level increase is accompanied by induction of oxidative stress response. As F5% increased ROS levels, the expression of the oxidative stress response genes was investigated. Nrf-2, the transcription factor, master inducer of expression of a multitude of oxidative stress response pathway genes12 including sod-1, an enzyme responsible for conversion of superoxide into less toxic hydrogen peroxide and oxygen23 were evaluated through RT-PCR. Only basal levels of both nrf-2 and sod-1 were expressed upon F5% treatment, the same as in control (Fig. 4). This lack of induction of oxidative stress response despite increased ROS may be the cause for motor neuronal cell death and other detrimental effects like vacuolation.
Discussion
Reports of chronic, indiscriminate high fructose consumption being linked to major neurological/ND including ALS24 are unsettling. ALS is more prevalent among sports personnel. Energy beverages consumed by them as an immediate source of muscular energy are supplemented with fructose. But fructose's effect on motor neuron degeneration in general, sports people and ALS is unclear. Hence, we set out to address fructose impact on the NSC-34 MN line in vitro.
In sports people, oxygen uptake is quite different, with high VO2max (maximal oxygen uptake).25 To closely mimic this, unlike normal mammalian cell culture, the oxygen level was maintained at 18.8%, with exclusive O2 supply in addition to 5% CO2. In vivo O2 level is below 6.5% in most of the tissues including brain and muscle.26 But that of the spinal cord is not known. Although F5% shuts off the energy metabolism machinery in the cells under said normal conditions of 37°C with 5%CO2 18 only through the combination of high oxygen and fructose, the negative effects of ∼85% cell death on day 10 (Fig. 1) through inhibition of cell division (Fig. 2) were observed. In addition, unexpectedly, around 1 to 2% of the cells differentiated with long, branched neurites and 0.5% of this populace were viable in vitro up to 80 days (Fig. 4), but only with O2 supplementation. Hence, even if high fructose combined with oxygen supplementation arrests cell proliferation, this study still provides a strategy for long-term maintenance of differentiated spinal cord MNs that is hard to achieve. A further detailed study will provide novel insights into the MN function and abnormalities on long term cultures.
Conclusion
High fructose is lethal to MNs (Figs. 1 and 2) due to excess ROS generation without oxidative stress response (Fig. 4) and complete abolition of mitochondrial activity.18 Unlike other cell types, as MNs lack regenerative ability after birth, and high fructose affects the differentiated MNs on the long term, the detrimentality brought about by F5% needs to be considered seriously. The only marginal benefit of high fructose is that it provides a strategy or direction, in combination with oxygen supplementation, to differentiate and maintain low percentage of MNs for a long term in vitro (Fig. 4) that is not possible otherwise. Overall, high fructose in diet has no benefit whatsoever and caution is required against its unfettered intake as part of the diet.
Acknowledgments
Dr Divya Lodha thanks Sri Ramachandra Institute of Higher Education and Research for the Chancellor's Fellowship. The authors thank the Center for Stem Cell and Regenerative Medicine and the Center for Indian System of Medicine, Sri Ramachandra Institute of Higher Education and Research for the infrastructure.
Conflict of Interest
None declared.
Funding D.L. and J.R.S. report all support from Sri Ramachandra Institute of Higher Education and Research for Chancellor's fellowship.
References
- Chronic fructose ingestion as a major health concern: is a sedentary lifestyle making it worse? A review. Nutrients. 2017;9(6):549.
- [Google Scholar]
- Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90(1):23-46.
- [Google Scholar]
- Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients. 2017;9(9):981.
- [Google Scholar]
- High-fructose diet during adolescent development increases neuroinflammation and depressive-like behavior without exacerbating outcomes after stroke. Brain Behav Immun. 2018;73:340-351.
- [Google Scholar]
- High-fructose intake as risk factor for neurodegeneration: key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol Dis. 2016;89:65-75.
- [Google Scholar]
- Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer's disease: metabolic basis for dementia. Sci Rep. 2016;6:27524.
- [Google Scholar]
- Contact sports as a risk factor for amyotrophic lateral sclerosis: a systematic review. Global Spine J. 2019;9(1):104-118.
- [Google Scholar]
- C9orf72 expansion within astrocytes reduces metabolic flexibility in amyotrophic lateral sclerosis. Brain. 2019;142(12):3771-3790.
- [Google Scholar]
- Fructose Promotes Leaky Gut, Endotoxemia and Liver Fibrosis through CYP2E1-Mediated Oxidative and Nitrative Stress. In: Hepatol. 2019. . Advance online publication
- [CrossRef] [Publisher] [Google Scholar]
- Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063-1075.
- [Google Scholar]
- Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401-426.
- [Google Scholar]
- An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14(6):1105-1116.
- [Google Scholar]
- Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat Neurosci. 2002;5(4):301-307.
- [Google Scholar]
- Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194(3):209-221.
- [Google Scholar]
- Secreted trophic factors of human umbilical cord stromal cells induce differentiation and neurite extension through PI3K and independent of cAMP pathway. Ann Neurosci. 2015;22(2):97-106.
- [Google Scholar]
- ALS associated mutant SOD1 impairs the motor neurons and astrocytes and wild type astrocyte secreted-factors reverse the impaired motor neurons. Ann Neurosci. 2011;18(2):48-55.
- [Google Scholar]
- Detrimental effects of fructose on mitochondria in mouse motor neurons and on C. elegans healthspan. Nutr Neurosci 2020 (ePub ahead of print)
- [CrossRef] [Google Scholar]
- Differential staining of DNA and RNA in intact cells and isolated cell nuclei with acridine orange. Methods Cell Biol. 1990;33:285-298.
- [Google Scholar]
- Anti-glycation and anti-oxidative effects of a phenolic-enriched maple syrup extract and its protective effects on normal human colon cells. Food Funct. 2017;8(2):757-766.
- [Google Scholar]
- Fructose promotes the differentiation of 3T3-L1 adipocytes and accelerates lipid metabolism. FEBS Lett. 2014;588(3):490-496.
- [Google Scholar]
- Hyperoxia in cell culture. A non-apoptotic programmed cell death. Ann N Y Acad Sci. 1999;887:164-170.
- [Google Scholar]
- ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020;37:101696.
- [Google Scholar]
- Peripheral nerve protein glycation and muscle fructolysis: evidence of abnormal carbohydrate metabolism in ALS. Funct Neurol. 1993;8(1):33-42.
- [Google Scholar]
- Oxygen uptake kinetics as a determinant of sports performance. Eur J Sport Sci. 2007;7:63-79.
- [Google Scholar]
- Oxygen and mammalian cell culture: are we repeating the experiment of Dr. Ox? Nat Metab. 2019;1(9):858-860.
- [Google Scholar]






