Translate this page into:
Hemodynamic effects of dexmedetomidine during intra-operative electrocorticography for epilepsy surgery
Address for correspondence: Dr. A Arivazhagan, Department of Neurosurgery, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, Karnataka, India. E-mail: arivazhagan.a@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Dexmedetomidine, a predominant alpha-2-adrenergic agonist has been used in anesthetic practice to provide good sedation. The drug is being recently used in neuroanesthesia during awake surgery for brain tumors and in functional neurosurgery.
Materials and Methods:
This prospective study analyzed the hemodynamic effects of dexmedetomidine infusion during electrocorticography in patients undergoing surgery for mesial temporal sclerosis. Dexmedetomidine infusion was administered during intra-operative electrocorticography recording, 15 minutes after the end tidal MAC of N2O and isoflurane were decreased to zero. Anesthesia was maintained with O2 : air mixture = 50:50, vecuronium and fentanyl. Heart rate (HR), mean arterial pressure (MAP) and end tidal carbon dioxide (ETCO2) were recorded across at induction, 2 min prior to dexmedetomidine (PreDEX), 5 min during dexmedetomidine infusion (DEX; 1 μg/kg), 5 min after stopping dexmedetomidine and 10 minutes after stopping dexmedetomidine.
Results:
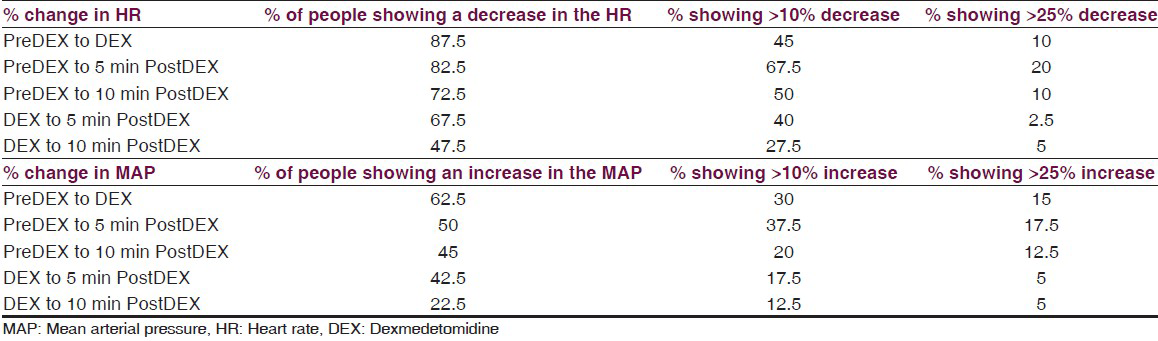
Forty patients with mesial temporal sclerosis (M: F = 27:13, mean age = 28.15 ± 10.9 years; duration of epilepsy = 12.0 ± 7.9 years) underwent anterior temporal lobe resection with amygdalohippocampectomy for drug-resistant epilepsy. Infusion of dexmedetomidine caused a transient fall in HR in 87.5% of patients and an increase in MAP in 62.5% of patients, which showed a tendency to revert back towards PreDEX values within 10 min after stopping the infusion. Sixty-five percent of the patients showed ≤25% reduction and 10% of them showed >25% reduction in HR. 47.5% of the patients showed ≤25% increase and 15% of them showed >25% increase in MAP. These changes were over a narrow range and within physiological limits.
Conclusion:
The infusion of dexmedetomidine for a short period causes reduction of HR and increase in MAP in patients, however the variations are within acceptable range.
Keywords
Dexmedetomidine
heart rate
hemodynamic changes
mean arterial pressure
Introduction
Dexmedetomidine (Dex) is an alpha-2 adrenergic agonist which has gained popularity in neurosurgery due to its sympatholytic action and nociceptive properties.[1] It is being increasingly used as a sedative during awake craniotomy and as well as in intensive care unit settings. Dex along with remifentanil and various other anesthetics such as ketamine, midazolam and propofol are being used for recording evoked potentials, microelectrode recordings, cortical stimulation etc,.[234] In all these techniques, it has been found that most anesthetics have a suppressant action because of their gamma aminobutyric acid (GABA)-mediated action. Most anesthetics during microelectrode recordings (MER) in DBS too cause similar difficulties, as the sub-cortical structures are extremely sensitive to effects of GABA.[56] However, Dex through its GABA-independent action provides well-preserved intraoperative MER and evoked potentials.[56]
Some studies have addressed the use of Dex in epilepsy surgery. The main agents used routinely for awake craniotomy with electrocorticography (ECoG) include propofol, midazolam–fentanyl combination and remifentanil.[7] During the whole procedure, the important aims are to provide adequate ventilation, maintain hemodynamic stability, adequate analgesia to ensure proper participation of the patient in the functional assessment and maintaining normal intracranial pressure (ICP). As the drug is being used increasingly in neurosurgical practice, it is important to establish the hemodynamic safety during the time the patients receive Dex.
In this study we have evaluated the effect of short-term bolus infusion of Dex intraoperatively in patients with drug-resistant temporal lobe epilepsy undergoing ECoG during anterior temporal lobectomy and amygdalohippocampectomy.
Materials and Methods
This prospective study was carried out with the approval of the institutional ethics committee in a tertiary neuroscience center. Patients with drug-resistant epilepsy due to mesial temporal sclerosis (MTS) who were planned for anterior temporal lobectomy and amygdalohippocampectomy were selected. A written informed consent was obtained from all the patients who were enrolled into the study. It is our institutional practice to record ECoG in all epilepsy surgery cases with focal lesions, including MTS. All cases of MTS undergo standard anterior temporal lobectomy and amygdalohippocampectomy irrespective of ECoG findings. We selected patients with MTS to obtain a homogenous cohort with an adequate sample size, since MTS is the most common focal pathology with drug-resistant epilepsy and managed by surgical resection. The ECoG findings in the study are being reported elsewhere. Patients were monitored during surgery with electrocardiogram (ECG), non-invasive blood pressure (NIBP – Datex Ohmeda S/5®), pulse oximetry, temperature, inspired and expired anesthetic concentration and capnography using an AS/5® monitor (GE health care, Helsinki, Finland). The cohort had stable hemodynamic parameters during the start of the study, unlike people undergoing neurosurgical procedures for raised ICP features or intrinsic brainstem lesions.
Intra operative procedure
Anesthesia was induced with intravenous fentanyl 2 μg/kg and propofol 2-2.5 mg/kg. Induction heart rate (HR) and mean arterial pressure (MAP) were recorded. Vecuronium 0.1 mg/kg was used to facilitate endotracheal intubation and lidocaine 1mg/kg was used to blunt the hemodynamic response to laryngoscopy and intubation. The patients’ lungs were mechanically ventilated with N2O:O2 and 1% isoflurane to maintain a PaCO2 at 30-35 mmHg. Intermittent doses of fentanyl and vecuronium were administered to maintain analgesia and skeletal muscle paralysis. Depth of anesthesia was measured using entropy/bispectral index which were maintained in the range of 40-50 throughout.
Five minutes prior to recording the ECoG, 1 μg/kg body weight of fentanyl and a dose of muscle relaxant were administered to prevent any movement during the recording. Isoflurane was stopped and the patient was ventilated with air: Oxygen mixture to allow the end tidal isoflurane concentration to fall to zero. The HR and MAP were recorded before starting Dex infusion (PreDex stage). Following this, all the patients were uniformly given a bolus dose of 1 μg/kg body weight of Dex intravenously over 5 minutes using an infusion pump (Fresnius Kabi, Base Primea) and the HR and MAP were recorded during the 5 min of infusion, during which ECoG was recorded. The recording was further continued for 10 min after stopping Dex and the values of the variables were noted at 5 min and 10 min after the infusion, as the effect of the drug was wearing off.
The hemodynamics of the patient was recorded through all the five stages of the study, namely stage 1-induction (IND), stage 2-before Dex infusion (PreDEX), stage 3-during Dex infusion (DEX), stage 4-5 min after the infusion (5 min PostDEX) and stage 5-10 min after the infusion (10 min PostDEX). End tidal carbon dioxide (ETCO2) was recorded during stages 2-4. After the ECoG recording, nitrous oxide and isoflurane were restarted and the surgical procedure was carried out. The changes in the hemodynamic parameters were analyzed offline. The changes in hemodynamic parameters during various stages of Dex were analyzed. The measures of central tendency and variation across the five stages are expressed as Mean ± Standard Deviation. A univariate analysis of variance (ANOVA) unassuming variances was used to evaluate the changes across stages. Since not all patients showed the same response across the stages, a cumulative frequency analysis was done to determine how many patients showed a differential response. A P < 0.05 was considered statistically significant.
Results
Forty patients were recruited in this study to analyze the effects of the drug during surgery. HR and MAP were monitored under Dex infusion in these patients. The mean age of the patients was 28.15 ± 10.9 years. The cohort comprised of 27 males and 13 females. All patients had MTS and underwent anterior temporal lobectomy and amygdalohippocampectomy for drug-resistant mesial temporal lobe epilepsy (right: 21, left: 19). The demographic details of the patient cohort are given in Table 1. The ETCO2 was maintained at around 30mmHg (PreDEX: 30.35, DEX: 30.1, PostDEX 5 min: 29.825, PostDEX 10 min: 29.425) during the procedure. The change in the measured parameters was compared across the stages using repeated measures ANOVA.

HR analysis
The mean HR increased from the time of induction (IND) to PreDEX but it was not statistically significant (P = 0.104). This could be because of the various stimuli such as intubation, craniotomy, etc., Interestingly, we noted that there is a significant decrease in the HR from PreDEX to DEX stage (P < 0.0001). This fall in the HR continued into the early post DEX stage (PostDEX 5 min) (P = 0.009) [Table 2, Figure 1]. However, this hemodynamic change was very short lived and during the subsequent 5 min it showed a tendency toward returning to the baseline. These changes were within the physiological limits (HR range: 67.5-79.85bpm). On analyzing the cumulative frequency of this event, we noted that a reduction in HR occurred in most of the patients (87.5%) in the cohort. Sixty-five percent of the patients showed ≤25% reduction, while 10% of the patients showed >25% reduction in HR [Table 3]. There was a greater than 50% fall in the HR transiently in three subjects, while the mean baseline HR for these individuals was 86.6 bpm. However, during this change the MAP was maintained at a mean of 101 mmHg. Hence, no additional action was taken and as soon as the infusion was stopped the HR returned toward baseline.
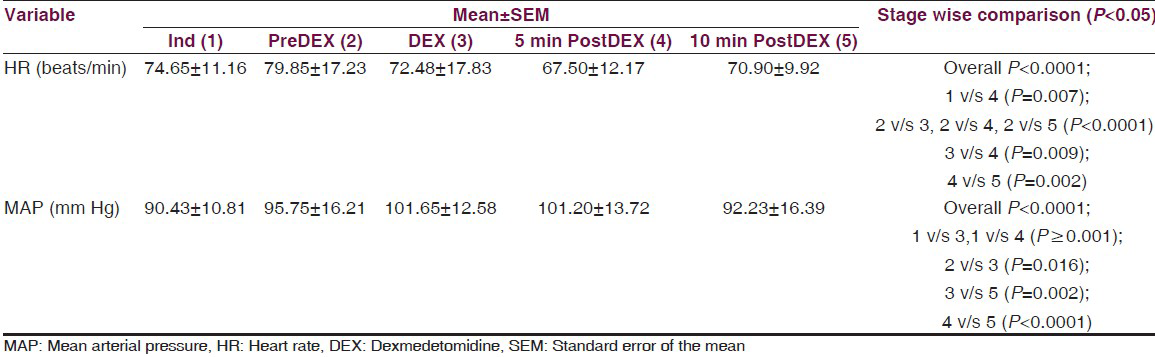
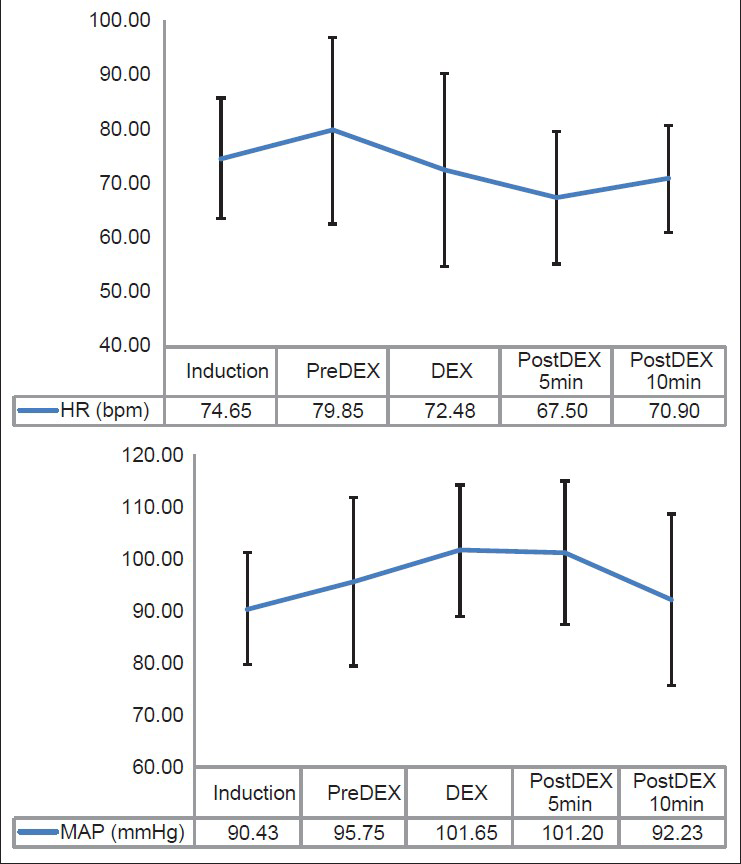
- The graph depicting the variations in average heart rate and mean arterial pressure in patients during dexmedetomidine infusion
MAP analysis
The MAP increased from the time of induction (IND) to PreDEX but the change was not statistically significant (P = 0.071). A significant increase in the MAP was noted from PreDEX to DEX stage (P = 0.016). 62.5% of the patients showed an increase in MAP from PreDex to Dex stage. While 47.5% of the patients showed ≤25% increase, 15% of them showed >25% increase in MAP [Table 3]. However, from DEX to post DEX 5 min stage, there was no significant change in MAP which remained elevated. During the subsequent 5 min it was noted that the MAP showed a tendency toward returning to the IND level which was significant (P < 0.0001) [Table 2, Figure 1]. Therefore, it was noted that there was an increase in the MAP during the time of Dex infusion which continues into the next 5 min after stopping the infusion. However, this hemodynamic change was very short lived and during the subsequent 5 min it showed a tendency toward returning to the PreDEX MAP. These changes were within the physiological limits (MAP range: 90.42-101.65 mmHg).
Discussion
Hemodynamic stability intraoperatively is very crucial for good postoperative outcome in neurosurgery. The hemodynamic complications include perioperative hypertension, intraoperative bradycardia and rarely asystole. Intraoperative hypertension can lead to increased bleeding at site, brain swelling through the durotomy making it very difficult for the surgeon to operate. Along with this, various respiratory complications have to be considered such as dose-dependent respiratory depression, a serious adverse effect while using thiopentone or propofol.[89]
The main effects of Dex are as a central sympatholytic and as a peripheral ganglionic blocker. This action is predominantly performed through agonistic action at the pre-synaptic alpha 2 adrenergic receptors. This causes a decrease in the release of catecholamines (both epinephrine and norepinephrine) into the synaptic junction.[101112] The initial response to rapid DEX infusion may be a transient hypertension.[1314] Dex has the characters of an ideal perioperative anesthetic and sedative agent, i.e. it does not cause respiratory suppression, provides good analgesia, rapid onset and short elimination half-life and a good anxiolytic effect. In contrast to benzodiazepines and other sedatives, patients treated with Dex are adequately sedated yet, arousable and responsive.
In this study, we evaluated a short duration use of Dex intra-operatively (lasting 5-10 min) in patients with drug-resistant temporal lobe epilepsy. A practical application is mainly in functional neurosurgeries such as epilepsy surgery, to identify the irritative zone intraoperatively without actually causing the awake state while maintaining stable hemodynamic parameters.
The hemodynamic changes were transient and within a very narrow range from the induction HR and MAP. This fall in HR is a noted effect with other drugs being used for epilepsy surgery such as propofol. Tarvainen et al. noted that there was an overall increase in HRV and decrease in HR prior to loss of consciousness noted with both Dex and propofol, but these changes were more considerable with Dex than for propofol.[15] This change has been noted to be beneficial intraoperatively as it has cardioprotective effect. An increase in the diastolic period with a marginal rise in blood pressure facilitates better cardiac perfusion, as myocardial perfusion tends to occur during the diastolic phase.[16] Post-operatively these patients recovered well and had no evident autonomic dysfunction at the time of discharge. Though studies have shown trends toward bradycardia (particularly in susceptible individuals) with prolonged use of Dex,[171819] it can be used safely for short duration anesthesia due to its rapid onset and wear off of its clinical effects. In our study, the effects wore off within 10 min after the infusion of the drug. However, it has been reported that the effects were more pronounced with longer duration of infusion. In a study conducted by Kabukcu et al., it was found that when a continuous infusion of Dex was administered at a dose of 1 μg/kg body wt/hour in patients undergoing coronary artery bypass surgery, it was found that the fall in the mean HR persisted even 10 min after stopping the infusion. Dex was continued at lower dose 0.2-0.4 μg/kg body wt/hour throughout the surgery, this change persisted into subsequent phases of the surgery. Interestingly, they noted an accompanying fall in MAP.[20] A simultaneous use of Dex along with other anesthetics such as fentanyl is also shown to produce a reduction in both the HR and systolic and diastolic blood pressures.[21] A fall in HR has been a consistent finding in most studies while the changes in blood pressure have been variable, along with a dose-dependent fall in blood pressure noted in one of the studies on healthy volunteers.[22] We noted that a reduction of HR occurred in most of the patients (87.5%), while an increase in MAP was less consistent. These hemodynamic effects are thought to be caused by biphasic action of Dex, i.e. a short lasting increase in MAP followed by a longer lasting hypotension because lower dosages reduce norepinephrine release, resulting in a decrease in vascular tone and hypotension, and higher dosages produce alpha-2B-mediated vasoconstriction and hypertension.[2324]
Conclusion
Dexmedetomidine was found to be a safe and hemodynamically stable drug in recording of electrocorticography during epilepsy surgery. The infusion of drug for a short period causes reduction of heart rate and increase in mean arterial pressure in patients, however the variations are within acceptable range.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg. 2008;107:1340-7.
- [Google Scholar]
- The effects of dexmedetomidine/remifentanil and midazolam/remifentanil on auditory-evoked potentials and electroencephalogram at light-to-moderate sedation levels in healthy subjects. Anesth Analg. 2006;103:1163-9.
- [Google Scholar]
- Use of dexmedetomidine and ketamine infusions during scoliosis repair surgery with somatosensory and motor-evoked potential monitoring: A case report. AANA J. 2011;79:89-90.
- [Google Scholar]
- Dexmedetomidine and arousal affect subthalamic neurons. Mov Disord. 2008;23:1317-20.
- [Google Scholar]
- Anesthesia for functional neurosurgery: The role of dexmedetomidine. Curr Opin Anaesthesiol. 2008;21:537-43.
- [Google Scholar]
- Motor and somatosensory evoked potentials are well maintained in patients given dexmedetomidine during spine surgery. Anesthesiology. 2008;109:417-25.
- [Google Scholar]
- A neuronal mechanism of propofol-induced central respiratory depression in newborn rats. Anesth Analg. 2004;99:49-55.
- [Google Scholar]
- The effect-site concentration of propofol producing respiratory depression during spinal anesthesia. Korean J Anesthesiol. 2011;61:122-6.
- [Google Scholar]
- The mechanism of alpha2-adrenergic inhibition of sympathetic ganglionic transmission. Anesth Analg. 1998;87:503-10.
- [Google Scholar]
- Pharmacodynamics of alpha2-adrenoceptor agonists. Best Practice and Research: Clinical Anaesthesiology. 2000;14:271-83.
- [Google Scholar]
- The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834-9.
- [Google Scholar]
- Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134-42.
- [Google Scholar]
- Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146-65.
- [Google Scholar]
- Analysis of heart rate variability dynamics during propofol and dexmedetomidine anesthesia. Conf Proc IEEE Eng Med Biol Soc 2010 2010:1634-7.
- [Google Scholar]
- Shortening deactivation of cardiac muscle: Physiological mechanisms and clinical implications. J Investig Med. 1999;47:369-77.
- [Google Scholar]
- Dexmedetomidine: An adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3:475-83.
- [Google Scholar]
- A randomized, double-blind, placebo-controlled dose range study of dexmedetomidine as adjunctive therapy for alcohol withdrawal. Crit Care Med. 2014;42:1131-9.
- [Google Scholar]
- The use of dexmedetomidine in anesthesia and intensive care: A review. Curr Pharm Des. 2012;18:6257-65.
- [Google Scholar]
- Hemodynamics in coronary artery bypass surgery: Effects of intraoperative dexmedetomidine administration. Anaesthesist. 2011;60:427-31.
- [Google Scholar]
- Hemodynamic effects of dexmedetomidine-fentanyl vs. nalbuphine--propofol in plastic surgery. Middle East J Anesthesiol. 2012;21:553-7.
- [Google Scholar]
- Hemodynamic characteristics of midazolam, propofol, and dexmedetomidine in healthy volunteers. J Clin Anesth. 2011;23:218-23.
- [Google Scholar]
- Insight into the effects of dexmedetomidine on intraoperative hemodynamics and postanesthetic recovery speed. Korean J Anesthesiol. 2012;62:111-2.
- [Google Scholar]
- Effects of low and high plasma concentrations of dexmedetomidine on myocardial perfusion and cardiac function in healthy male subjects. Anesthesiology. 2006;105:902.
- [Google Scholar]






