Translate this page into:
Hansen's Neuritis Revisited – A Clinicopathological Study
Address for correspondence: Dr. Shrijeet Chakraborti, Department of Pathology, Kasturba Medical College, Lighthouse Hill Road, Mangalore - 575 001, Karnataka, India. E-mail: shrijeet_chak@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Leprosy affecting the nerve solely or with concomitant skin lesions is not an uncommon condition in clinical practice. It is responsible for extensive morbidity and often poses a diagnostic challenge. This study aims to highlight the clinicopathological features of Hansen's neuritis (HN).
Materials and Methods:
In this retrospective study, cases of histologically diagnosed HN, from January 2010 to July 2017, were reviewed in the light of clinical features, treatment history, and outcome.
Results:
There were 18 cases of HN which accounted for 3.97% of total nerve biopsy samples (n = 453) and 0.02% of total histopathology samples (n = 81,013). The male: female ratio was 5:1 in the cases of HN. Age range was 20–79 years with a mean age of 42.4 years (standard deviation: ±14.03). Among the HN cases, there were 13 cases of pure neuritic leprosy (61.1%). Mononeuritis multiplex was the most common finding in the nerve conduction study. Six (33.3%) cases exhibited histological features of borderline tuberculoid leprosy, followed by five (27.8%) cases of mid-borderline features, three (16.7%) cases each of borderline lepromatous and burnt-out HN, and one (5.6%) case of polar tuberculoid leprosy. Lepra bacilli were detected on Fite-Faraco stain in 44.4% cases.
Conclusion:
Diagnosis of HN depends on astute search for skin lesions, nerve thickening or tenderness, sensory or motor symptoms, histopathological examination, and demonstration of lepra bacilli.
Keywords
Hansen's neuritis
lepra bacilli
mononeuritis multiplex
pure neuritic leprosy
INTRODUCTION
Hansen's disease is a chronic granulomatous disorder affecting the skin and nerves and is caused by Mycobacterium leprae. The earliest classification of this disease based on immunity status and response was given by Ridley and Jopling in 1966.[1] Wade in 1952 first proposed the disease “neural leprosy,” in which only peripheral nerves were involved. The technical committee of International Leprosy Congress in 1953, held in Madrid, accepted “neuritic leprosy” as one subtype among the major groups of leprosy.[2] Neuritic leprosy is one of the important causes of inflammatory peripheral neuropathy worldwide, especially in the tropical countries. In the absence of skin involvement, pure neuritic leprosy (PNL) may clinically mimic vasculitic neuropathy. In this study, we describe the clinicopathological profile of Hansen's neuritis (HN) diagnosed in our institute.
MATERIALS AND METHODS
The study was conducted in the Department of Pathology, Kasturba Medical College, Mangalore, Karnataka, India. Institutional Ethical Committee's permission was obtained before commencing this study. We retrieved clinicopathological data and slides of all the histologically diagnosed cases of HN from our archives, during the period from January 2010 to July 2017. The samples were received in the department from the parent institute, other medical colleges, and hospitals in and around Mangalore. All the cases were microscopically reviewed taking note of clinical details, including findings of nerve conduction study (NCS), which was collected from the medical records department and biopsy requisition forms. The corresponding skin biopsies were also reviewed.
Formalin-fixed, paraffin-embedded transverse and longitudinal sections of nerve segments were stained with hematoxylin and eosin, Masson trichrome, and Fite-Faraco (Fite) stains. Simultaneously, the transverse sections were also stained with Kulchitsky Pal (KPal)stain (for myelin), after secondarily fixing of the nerve biopsies in Fleming's solution. In the nerve and skin biopsies, the bacillary index (BI) was computed based on Ridley's scoring scheme.[3] Microscopic pathology in the nerve was categorized into indeterminate[4] and burnt-out/healed Hansen's categories as well as in addition to the five classes of Ridley and Jopling scheme.
All the demographic data, clinical findings, and results of microscopic examination were tabulated and analyzed by mean, standard deviation (SD), and percentages. The results were laid out in the form of graph and tables. Statistical methods could not apply in this study because of small sample size. Sincere attempts were made to collect the follow-up data in all the cases.
RESULTS
In the above-mentioned period, a total number of 81,013 samples were received in the pathology department for histopathological examination, of which there were 1982 skin biopsies (2.45% of total histopathology samples). Hansen's disease of the skin was diagnosed in 131 (6.6% of total skin biopsies and 0.16% of total histopathology cases). During the same time span, 453 nerve biopsies (0.56% of total histopathology samples) were received for etiological diagnosis of peripheral neuropathy. HN was diagnosed in 18 (3.97% of total nerve biopsy samples and 0.02% of total histopathology samples).
Sural nerve was biopsied in 16 cases, followed by right greater auricular nerve and cutaneous branch of left radial nerve in one case each. The male: female ratio was 5:1 in the cases of HN with 15 (83.3%) cases diagnosed in men. Age range was 20–79 years with a mean age of 42.4 years (SD ± 14.03). Maximum number of male patients presented in the age group of 41–50 years (5 cases, 27.8%), followed by three cases (16.7%) each in the age groups of 21–30 years and 31–40 years, two cases (11.1%) in 51–60 years, and one case (5.6%) each in the age groups of 11–20 years and 71–80 years. The three female cases belonged to the age groups of 31–40 years, 41–50 years, and 51–60 years (5.6% each) [Table 1].

Skin lesions consistent with leprosy were present in 5 out of 18 cases (27.78%) of HN, and in other 2 cases, there was no information available on skin affection by lepra bacilli. Hence, there were 13 cases of PNL (61.1%) among the HN cases, and these accounted for 2.8% cases of all nerve biopsy (n = 453) samples. Case 1 had a single hypopigmented and hypoesthetic skin patch in left buttock, and Case 4 had multiple hypopigmented macules in the trunk and erythematous patches in both the legs. Histopathological examination of the skin biopsies in Cases 1 and 4 revealed borderline tuberculoid (BT) (BI: zero) and borderline lepromatous (BL) (BI: 5) morphology, respectively. The corresponding nerve biopsies in both these cases showed BB morphology. Cases 11, 16, and 18 also had skin lesions which were not biopsied. Age range in PNL was 20–56 years with a mean age of 40.6 years (SD ± 10.78). Maximum number of male patients was in the age group of 41–50 years (4 cases; 30.7%), followed by two cases (15.4%) each in the age groups of 21–30 years, 31–40 years, and 51–60 years, and one case (7.7%) in the age group of 11–20 years. One female case (7.7%) each presented in the age groups of 31–40 years and 41–50 years.
The patients had myriad signs and symptoms. Most of the cases (44.4%; 8 out of 18 cases) presented with features of sensory loss and motor weakness with variation in spatial and temporal distribution. In 4 cases (22.2%), one or multiple peripheral nerves were thickened. Three cases (16.7%) had nonhealing trophic ulcers in the foot. Two (11.1%) patients were alcoholics and one case (5.6%) had retroviral disease. Two (11.1%) cases each had clinical history of peripheral neuropathy and mononeuritis multiplex. Case 1 presented with lower motor neuron palsy of bilateral facial nerves of 5 days duration [Figure 1], involving only the upper fibers, and Case 18 had claw hand for 2 years. Rheumatoid factor positivity was noted in Case 6, and there was family history of leprosy in skin in Case 14. There were no clinical details available in Case 9 [Table 1].
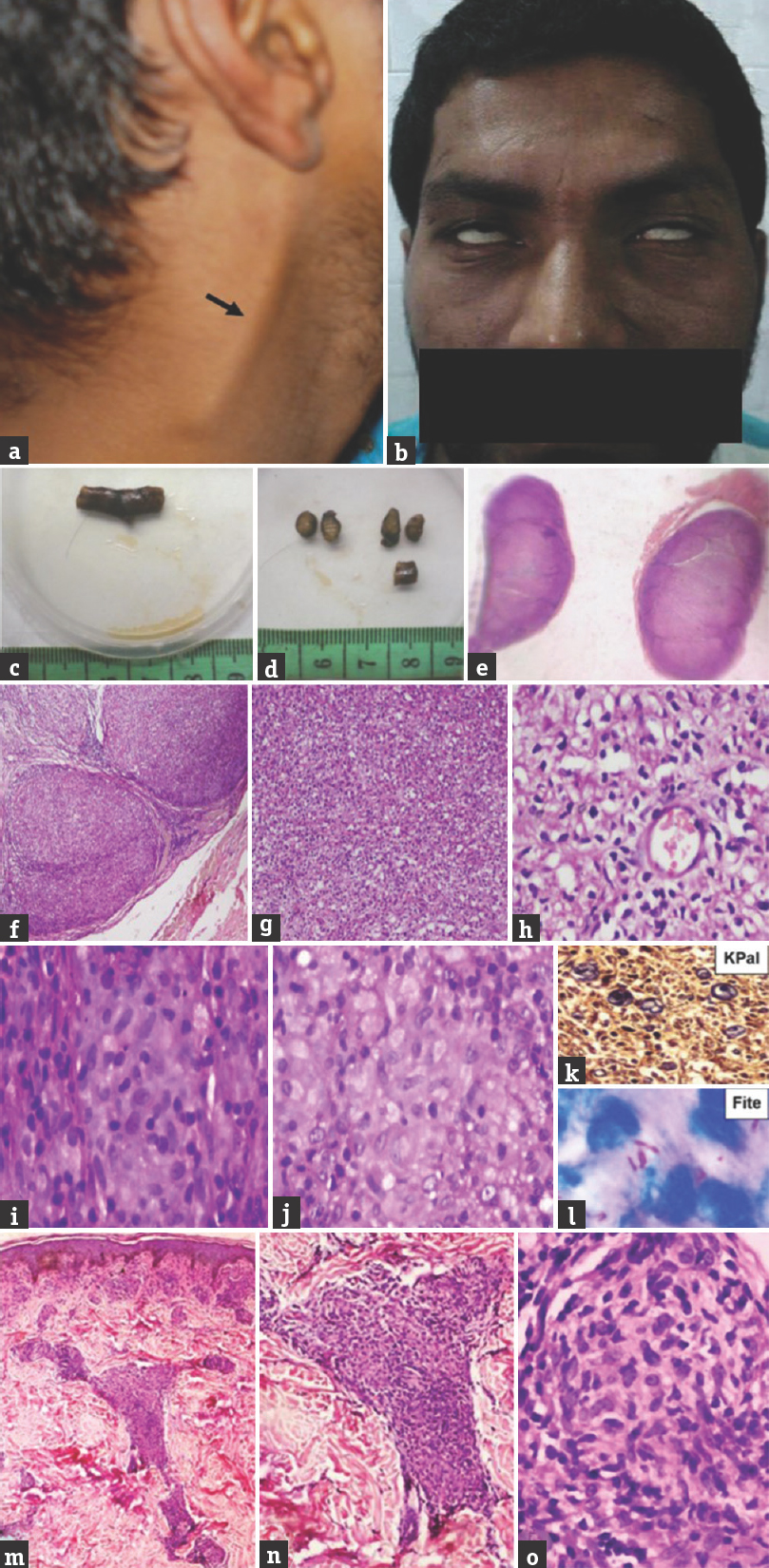
- Case 1 mid-borderline – (a) Thickened right greater auricular nerve; (b) bilateral lower motor neuron facial palsy exhibiting lagophthalmos; (c and d) gross of thickened right greater auricular nerve; (e) whole-mount view of transverse section of thickened nerve; (f) enlarged fascicles (×40, H and E); (g) fascicles completely effaced by inflammatory cells (×100, H and E); (h) mononuclear cells, foam cells, and histiocytes (×200, H and E); (i and j) poorly formed granuloma (×400, H and E); (k) few remnant myelinated nerve fibers (×400, KPal); (l) lepra bacilli (OIF, Fite); (m) (×40, H and E), (n) (×100, H and E), (o) (×400, H and E); well-defined epithelioid granuloma in papillary and reticular dermis
The findings of NCS available in 13 (72.2%) cases revealed a common pattern of mononeuritis multiplex with severe degree of sensory more than motor axonopathy. In Case 14, NCS recorded axonal neuropathy involving the bilateral median, right superficial peroneal, saphenous, sural, and posterior tibial nerves. Seven cases had a clinical diagnosis of HN, of which three cases had differential diagnoses of Charcot-Marie-Tooth disease, vasculitic neuropathy and diabetic neuropathy. In one (5.6%) case each, the provisional diagnosis was burnt-out HN, vasculitic neuropathy, rheumatoid vasculitis, and nutritional neuropathy. Provisional clinical diagnosis was not available in seven (38.9%) cases [Table 1].
In the nerve biopsies examined, six (33.3%) cases exhibited histological features of BT leprosy [Figure 2], followed by five (27.8%) cases of mid-borderline (BB) features [Figure 1], three (16.7%) cases each of BL [Figure 3] and burnt-out HN [Figure 4], and one (5.6%) case of polar tuberculoid (TT) leprosy [Figure 5]. There was no case of polar lepromatous (LL) or indeterminate morphology among the cases studied. Among the HN cases which had concomitant skin lesions, there were two cases of BB (Case 1 and 4) and healed HN each (Case 16 and 18), and one case (Case 11) BL. There were six cases having BT histology, followed by three cases of BB, two cases each of BL, and one case each of burnt-out HN and TT morphology among the 13 PNL cases.

- Case 3 borderline tuberculoid – (a) Distinct inflammation in endo- and perineurium, marked endoneurial fibrosis (inset, Masson trichrome) and severe loss of myelinated nerve fibers (×40, H and E); (b) thickened perineurium and epineurial perivascular inflammation (×100, H and E); (c) thickened perineurium and endoneurial inflammation (×100, H and E); (d) epithelioid granuloma (×400, H and E)
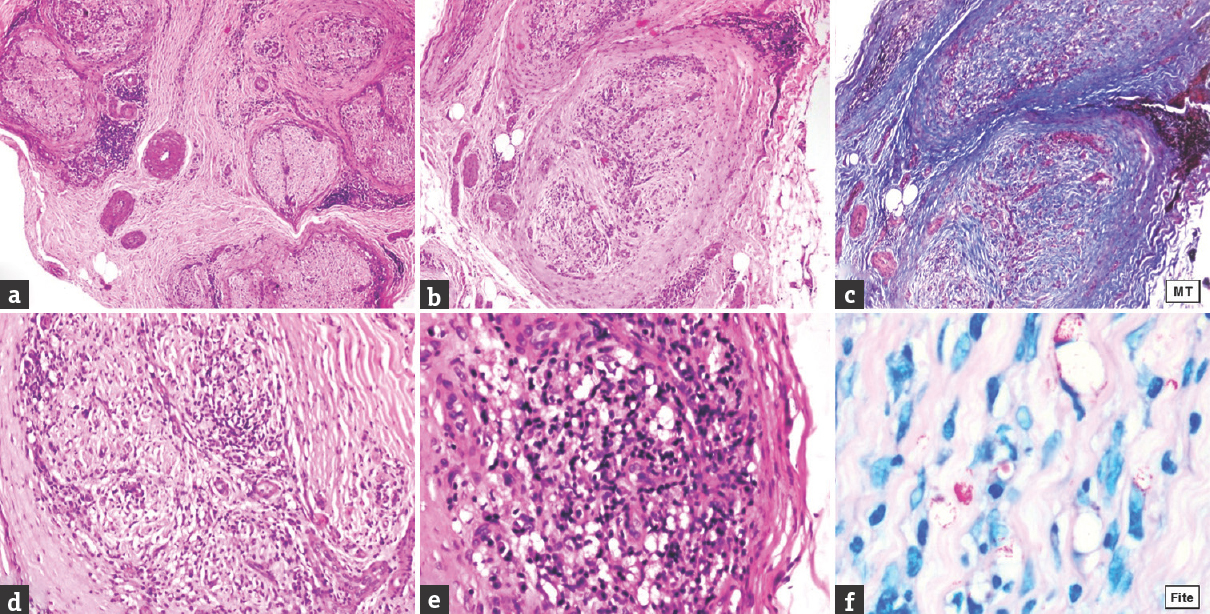
- Case 5 borderline lepromatous – (a) Inflammation in endo-, peri-, and epineurium (×40, H and E); (b) thickened perineurium (×100, H and E); (c) marked endoneurial fibrosis and severe loss of myelinated nerve fibres (×100, Masson trichrome); (d) endoneurial inflammation (×200, H and E); (e) dense lymphocytic infiltrate admixed with foam cells (×400, H and E); (f) numerous lepra bacilli and globi (OIF, Fite)
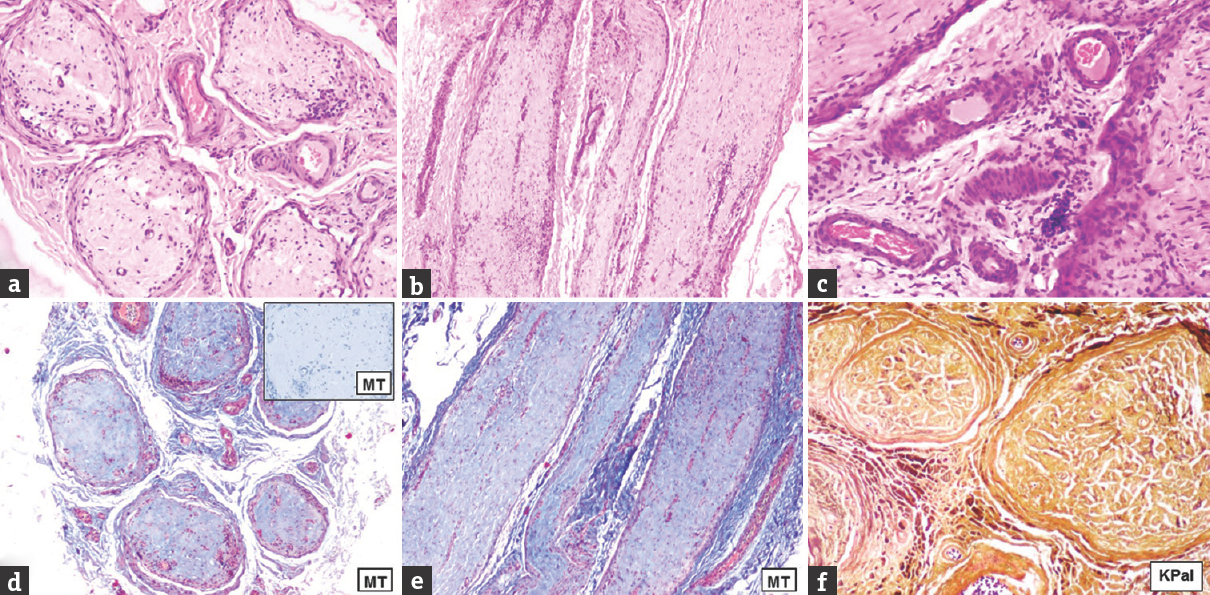
- Case 8 burnt-out Hansen's – (a) (×40, H and E), (b) (×100, H and E): mild lymphocytic infiltrate in endo-, peri-, and epineurium; (c) neovascularization and mild perivascular lymphocytic infiltrate in epineurium (×400, H and E); (d) (×40, Masson trichrome), (d) inset, (e) (×100, Masson trichrome): Dense collagenization in the endoneurium; (f) complete loss of myelinated nerve fibers (×100, KPal)
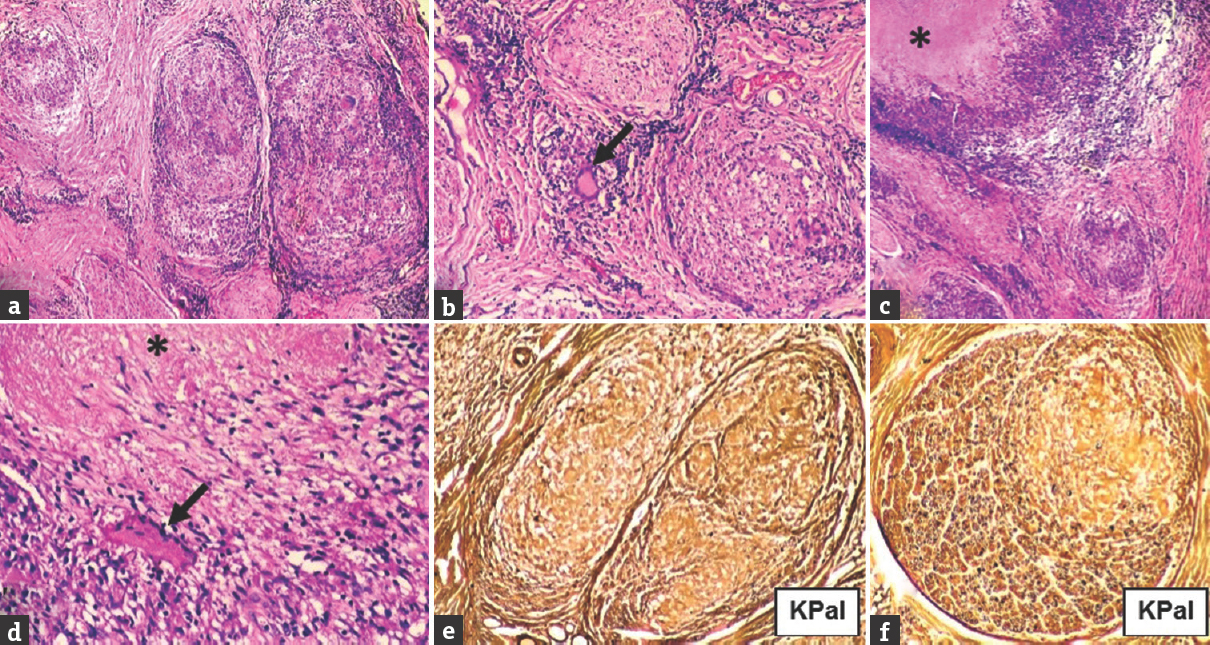
- Case 15 polar tuberculoid – (a) Enlarged fascicles with moderate endo-, peri-, and epineurial inflammation, and epithelioid granulomas (×40, H and E); (b) granuloma with Langhans giant cell (arrow) in epineurium (×200, H and E); (c) caseating (asterix) epithelioid granuloma (×40, H and E); (d) caseating (asterix) epithelioid granuloma and Langhans giant cell (arrow) (×400, H and E); (e) complete loss of myelinated nerve fibers (×200, KPal); (f) moderate degree loss of myelinated nerve fibres (×200, KPal)
Complete loss of myelinated fiber loss was seen in 12 (66.7%) cases and among them four cases showed BT morphology, followed by three cases each of BB and burnt-out morphology, and two cases of BL morphology. Severe degree of myelin loss was noted in four (22.2%) cases, and among them two cases had BT, and one case each had BB and TT morphology. Two (11.1%) cases (BL and BB morphology) had moderate degree of myelinated fiber loss. Among six cases of BT leprosy in the nerves, three (50%) cases exhibited well-defined endo-, peri-, and endoneurial epithelioid granulomas, whereas endo- and perineurial epithelioid granulomas were seen in two (33.33%) cases and only endoneurial granulomas in one (16.67%) case. Among five cases of BB leprosy in the nerves, three (60%) cases exhibited poorly defined granulomas involving only the endoneurial compartment, one (20%) case each involving all three and both endo- and perineurial compartments. The only case of TT showed necrotizing epithelioid granulomas having Langhan's giant cells and rimmed by dense lymphoplasmacytic infiltrate, involving the endo-, peri-, and epineurium. Hence, epithelioid granulomas were detectable in 66.67% (12) cases. All three (100%) cases of BL did not show granulomas; rather, the fascicles were effaced by sheets of lymphohistiocytic infiltrate and interspersed foam cells.
All three (100%) cases of burnt-out HN were characterized by dense endoneurial collagenization or sclerosis, mild-to-moderate lymphohistiocytic inflammation in all the three compartments which was localized predominantly around blood vessels and thickened nutrient vessels. Foam cells were seen in eight (44.4%) cases, which included all cases of BL and BB, and two cases of BT. Plasma cells were present in all cases of BT and TT. Perineurial thickening was present in four (22.2%) cases of HN, which included two cases of BL, and one case each of BB and burnt-out HN. Out of 18 HN biopsies, epineurial perivascular inflammation, neovascularization, and vascular thickening were present in 17 (94.44%), 4 (22.22%), and 3 (16.67%) cases, respectively [Table 1]. There were not any microscopic features to differentiate between PNL and HN with skin lesions.
BI in HN cases ranged from 0 to 6 in five (83.33%) out of six cases of BT leprosy and three (100%) cases of burnt-out HN. One case each of TT (100%) and BL (33.3%) had a BI of zero. lepra bacilli were detected on Fite-Faraco stain in 8 (44.4%) out of 18 cases. BI was 1 and 4 in one case each of BT (16.7%) and BL (33.3%), respectively. Toward the lepromatous end of the spectrum, density of lepra bacilli in the tissue increased with a BI of 2 in 2 cases of BB, to BI-5 in 1 case each of BB and BL and BI-6 in 1 case each of BB and BL. Globi were encountered in 3 (16.67%) out of 18 cases, that is cases -5 (BL; BI-5), -11 (BL; BI-6), and -17 (BB; BI-6).
Out of 18 cases, 14 cases were classified as multibacillary (MB) and treated with multidrug therapy (MDT). In two cases, treatment history was not available and two cases of burnt-out HN did not receive any treatment. Complete recovery was seen in eight cases. There was resolution of sensory symptoms in two cases, persistence of motor weakness and foot drop in one case each. One case is still undergoing MB-MDT and three cases were lost to follow-up. Lepra reactions were not reported in any of the cases.
DISCUSSION
In 1903, Albert Neisser described a “neural type of leprosy/lepra nervorum” for the first time and added the same to the already accepted “nodular” and “anesthetic” forms of leprosy.[5] The Indian Association of Leprologists (IALs) included the distinct form of “neural leprosy” in their official six group classification in 1955 and named it “polyneuritic leprosy.”[5] HN clinically manifests as thickened or tender nerves leading to sensory, motor, or autonomic disturbances and formation of trophic ulcers as well as loss of tissue. World Health Organization (WHO) in 1997 classified Hansen's disease based on number skin lesions as paucibacillary (PB) (1 lesion), PB (2–5 lesions), and MB (>5 lesions).[6] Later, WHO categorized LL, BL, and BB cases of the Ridley–Jopling classification, with a bacteriological index of ≥2 at any site in the initial skin smears as MB leprosy. On the other hand, PB leprosy included indeterminate, TT, and BT with a bacteriological index of < 2. At least one of the cardinal signs is mandatory for the diagnosis of leprosy, which include definite loss of sensation in a pale (hypopigmented) or reddish skin patch, a thickened or enlarged peripheral nerve with a loss of sensation and/or weakness of the muscles supplied by that nerve, or the presence of acid-fast bacilli in a slit-skin smear. The cases can be classified as PB or MB based on number of skin lesions when slit-skin smear examination is not available. It is recommended that the patient should be treated as a MB Hansen's case whenever the classification of an individual case is in doubt.[7] There is the absence of any WHO guidelines for subclassification of PNL as PB or MB disease or its treatment.[8] In 1982, IAL re-christened “polyneuritic leprosy” as “pure neuritic type of leprosy.”[2] Single or multiple larger nerve trunks or their branches are enlarged in PNL with sensory loss in the corresponding dermatome, with neither skin lesions nor slit-skin smear positivity. Microscopy may reveal TT, borderline nonspecific, or even lepromatous morphology along with acid-fast bacilli.[2] According to the present National Leprosy Eradication Programme guidelines in India for therapeutic purpose, involvement of one or more nerve trunks is considered as PB and MB, respectively.[9]
In 2014, throughout the world, 213,899 people were newly diagnosed with Hansen's disease, which corresponds to a detection rate of 3.0/100,000 of population. Southeast Asian region registered the highest incidence of 8.12/100,000 of population. The overall registered prevalence in the world and Southeast Asia is 0.25 and 0.63/100,000 population, respectively. Of the newly diagnosed cases, 94% patients were reported in 13 countries: Bangladesh, Brazil, Democratic Republic of Congo, Ethiopia, India, Indonesia, Madagascar, Myanmar, Nepal, Nigeria, the Philippines, Sri Lanka, and the United Republic of Tanzania. MB cases constituted 61% of the patients, of which 74% were males.[10]
India contributes to more than 50% of new cases detected globally every year. In the year 2011–2012, 127,000 new cases were diagnosed with an annual new case detection rate of 10.35/100,000 of population. As on April 1, 2012, a total of 83,000 cases are on record with a prevalence rate of 0.68/10,000 population.[9] According to Indian data, PNL constituted about 4%–18% of leprosy patients.[11] The incidence is reportedly higher in South India comprising up to 18% of new cases. PNL is more common in men,[12] as in the present study. In the study by Mendiratta et al., most number of cases occurred in the age group of 15–30 years,[12] unlike the preponderance in 40–50 years revealed in the present study.
Among 46 cases of PNL, peripheral nerves were thickened in 32.6% cases, followed my trophic ulcers in 21.7% cases, muscle wasting, claw hand, and foot drop in 15.2%, 10.8%, and 8.7% cases, respectively.[13] Mononeuritis multiplex was the most frequent clinical and electrophysiological pattern of nerve dysfunction, with sensory (89% of all cases) more than motor (81%) dysfunction and predominant axonal neuropathy[14] comparable to the current study. Bilateral facial palsy is less common than unilateral facial palsy and the incidence of the same in leprosy varies from 3% to 24.59%.[15] Reddy et al. described dichotomy between immunological grading of skin and nerve lesions in cases where both are involved simultaneously, with the nerve showing lower spectrum of the disease,[16] and such a finding was noted only in case-4.
In the study by Hui et al. in 46 cases of PNL, there were 47.8% cases of BT, followed by 23.9%, 13%, and 10.8% cases of BL, TT, and BB, respectively.[13] Endoneurial, perineurial, and epineurial inflammation was seen in 39.8%, 39.8%, and 27.7% cases, respectively. Epithelioid granulomas were described in 13.2% cases. Fibrosis was most common (34.7% cases) in perineurial compartment, followed by endoneurial and epineurial compartments in 31.6% and 26.3% cases, respectively. Reduction in myelinated fibres was noted in 55.6% cases. Mononuclear infiltrate and foamy macrophages were encountered in 42.3% and 19% cases. Endoneurial edema was described in 7.8% cases. In 19% cases, perineurial enlargement was noted. Microfascicles are nest-like structures composed of a variable quantity of small myelinated or nonmyelinated fibres and Schwann cells and surrounded by perineurial cells. These structures were discerned in 10.42% samples. In the same study, mononuclear cell infiltrate and fibrosis were seen in all three compartments in almost all the cases, epithelioid granulomas in 66.7% cases, foam cells in 44.4% cases, and perineurial thickening in 33.3% cases[17] Microfascicles were not seen in any of the cases in the present study. Another study reported epithelioid granulomas in 14% cases.[13] Reduction in myelinated fibres was noted in 55.6% cases,[16] unlike moderate-to-complete degree of myelinated fiber loss in all the cases in this study. Loss of myelinated fiber and Schwann cells is consistent with decrease in immunohistochemical staining of neurofilament in 100% HN biopsies, nerve growth factor receptor loss in 81.8%, PGP 9.5 staining loss in 100%, and loss of S-100 as well as myelin basic protein immunoreactivity in 90.9% cases each.[18] Caseous necrosis was recorded in one case of TT (case-15; present study) as was reported by Hui et al.[13]
Tests for Hansen's disease include slit-skin smears, skin and nerve biopsies, polymerase chain reaction (PCR), Lepromin test, and serum assays for phenolic glycolipid 1 antibody (anti-PLG1).[19] Anti-PLG1 titer correlates with bacterial load, being higher in lepromatous than TT cases.[4] It can be useful for monitoring chemotherapy as titers correlate with the BI following treatment.[20] Antunes et al. demonstrated lepra bacilli in 36.1% (52 out of 144) cases by Fite-Faraco stain. In the Lepra-negative cases, diagnosis of PNL was based on the PCR detection of M. leprae (28 out of 92 cases) and positivity of anti-PLG1 in the rest.[17] In the present study, lepra bacilli were highlighted by Fite-Faraco stain in 44.4% cases, comparable to 47.8% in the study by Hui et al.[13] Other ancillary tests were not done for the cases reviewed in our study.
CONCLUSION
HN is a debilitating but a completely treatable disease. Mononeuritis multiplex is the most common clinical presentation. Histological features such as mononuclear inflammation and foam cells in all compartments of the peripheral nerve, epithelioid granulomas, dense fibrosis, and less commonly perineurial thickening, with or without positivity for lepra bacilli are clues to diagnosis of HN. Knowing the morphological spectrum of HN is of cardinal importance because of the curable nature of the disease. Last but not the least, HN is one of the few neurological diseases with idiosyncratic clinical features and therefore a diligent neurological examination along with nerve biopsy examination is qualified for.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-73.
- [Google Scholar]
- Pure neuritic leprosy: Current status and relevance. Indian J Dermatol Venereol Leprol. 2016;82:252-61.
- [Google Scholar]
- Bacterial indices. In: Cochrane RG, Davey TF, eds. Leprosy in Theory and Practice. Bristol: John Wright and Sons Ltd; 1964. p. :620-2.
- [Google Scholar]
- Prasad PV, ed. All about Leprosy. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2005.
- 1997. WHO Seventh Expert Committee; June. Available from: http://www.who.int/lep/resources/expert/en/index1.html
- World Health Organization. WHO Expert Committee on Leprosy: Eighth report. Geneva: World Health Organization; 2012. p. :11-2.
- [Google Scholar]
- National Leprosy Eradication Programme Training Manual for Medical Officer, Central Leprosy Division, Directorate General Of Health Services, Ministry of Health and Family Welfare (Government of India), Nirman Bhavan, New Delhi. 2013
- [Google Scholar]
- World Health Organization. Global Leprosy Strategy 2016-2020: Accelerating towards a Leprosy-Free world. Geneva: World Health Organization; 2016. p. :3-4.
- [Google Scholar]
- Leprosy: Classification and clinical aspects. In: Valia RG, Valia AR, eds. IADVL Text Book of Dermatology (3rd ed). Mumbai: Bhalani Publishing House; 2008. p. :2032-69.
- [Google Scholar]
- Primary neuritic leprosy: A reappraisal at a tertiary care hospital. Indian J Lepr. 2006;78:261-7.
- [Google Scholar]
- Pure neuritic leprosy: Resolving diagnostic issues in acid fast bacilli (AFB)-negative nerve biopsies: A single centre experience from South India. Ann Indian Acad Neurol. 2015;18:292-7.
- [Google Scholar]
- Leprosy of the oral cavity and adnexa. Oral Surg Oral Med Oral Pathol. 1962;15:1178-94.
- [Google Scholar]
- A comparative evaluation of skin and nerve histopathology in single skin lesion leprosy. Indian J Dermatol Venereol Leprol. 2005;71:401-5.
- [Google Scholar]
- Histopathological examination of nerve samples from pure neural leprosy patients: Obtaining maximum information to improve diagnostic efficiency. Mem Inst Oswaldo Cruz. 2012;107:246-53.
- [Google Scholar]
- An immunohistochemical, clinical and electroneuromyographic correlative study of the neural markers in the neuritic form of leprosy. Braz J Med Biol Res. 2006;39:1071-81.
- [Google Scholar]
- Hansen disease in the United States in the 21st century: A review of the literature. Arch Pathol Lab Med. 2007;131:982-6.
- [Google Scholar]
- Detection of phenolic glycolipid I of Mycobacterium leprae in sera from leprosy patients before and after start of multidrug therapy. Clin Diagn Lab Immunol. 2001;8:138-42.
- [Google Scholar]






