Translate this page into:
Feasibility of utilizing augmented reality in neurosurgery: Insights from a single-center experience
*Corresponding author: Diego F. Gómez, Department of Neurosurgery, Hospital Universitario Fundación Santa Fé de Bogotá, Bogotá, Colombia. diegogomezncx@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gómez D, Vargas-Osorio M, RamírezSanabria AD, Villegas-Gomez GA, Ordonez-Rubiano E, Ramón J, et al. Feasibility of utilizing augmented reality in neurosurgery: Insights from a single-center experience. J Neurosci Rural Pract. doi: 10.25259/JNRP_454_2024
Abstract
Objectives
Augmented reality (AR) has recently evolved, offering unprecedented precision in the surgical management of brain tumors. AR devices empower surgeons to visualize patient anatomy by seamlessly integrating 3D-reconstructed scans into the surgical site, thus improving surgical precision and efficiency.
Materials and Methods
This retrospective case-series study analyzed cases operated on at a single center from January 2020 to June 2022. Patients underwent craniotomy with AR-guided neuronavigation. Pre-operative magnetic resonance imaging sequences were processed. AR software superimposed 3D virtual objects onto the surgical field.
Results
The study analyzed 14 brain lesion cases involving patients aged 11–79, with lesions in various anatomical locations, including the frontal lobes, petroclival area, and cavernous sinus. Pathologies ranged from glioblastomas and meningiomas to metastatic carcinomas. Patients underwent elective craniotomy with AR-guided neuronavigation to identify critical areas such as Broca’s and Wernicke’s areas, motor areas, and neurovascular structures. Gross-total or near-total resection was achieved in all cases, with surgical times ranging from 2 to 9 h. No intra- or post-operative complications were reported. Hospital stays varied from 2 to 13 days.
Conclusion
AR significantly enhanced surgical accuracy and patient safety by enabling precise identification of critical areas and structures. It improved resection extension and accuracy for various brain lesions, reducing neurovascular injuries while preserving neurological function. Future research should explore AR’s impact on clinical outcomes and continue advancing its applications in neurosurgery.
Keywords
Augmented reality
Image-guided surgery
Neuronavigation
Virtual reality
INTRODUCTION
Technological advancements in neurosurgery have historically occurred approximately every two decades.[1] A significant milestone in neurosurgery was the adoption of the surgical microscope in the late 1950s, which dramatically transformed neurosurgical procedures. More recently, virtual reality found its applications in neurosurgery in the 1990s.[1] Augmented reality (AR), a technology that overlays computer-generated images onto a user’s real-world view creates a combined perspective that enhances the user’s current perception of reality[2] and has been incorporated into neurosurgery in the past decade.[1-5] Recently, AR has advanced swiftly, providing unmatched precision in the surgical treatment of central nervous system tumors. AR devices allow surgeons to visualize patient anatomy by merging 3D-reconstructed computed tomography (CT) or magnetic resonance imaging (MRI) scans directly into the surgical area, a technique known as “in situ” visualization.[6] This method significantly enhances the surgeon’s perception by overlaying digital images onto the surgical field.[2]
The successful use of AR depends on precise alignment between patient imaging (usually CT or MRI) and the surgical site, which is essential for safe image-guided navigation. AR systems aim to enhance workflow in neurosurgical operating rooms by overlaying computer-generated 2D or 3D images onto the surgeon’s real-world view.[7,8] However, the application of AR in minimally invasive surgery presents unique challenges and may extend the surgical time. Given the high costs of the intraoperative implementation of AR, this technology has been largely implemented in high-income countries.[2] Our study provides information on the feasibility of using intraoperative AR in a low-to-middle-income country (LMIC). Our institution has integrated AR navigation for brain lesion resection by combining neuronavigation systems, robotic visualization, and advanced intraoperative imaging. This paper aims to present our initial experience and learning lessons using AR, highlighting the barriers and their potential benefits for neurosurgical procedures.
MATERIALS AND METHODS
This retrospective study analyzed a prospectively acquired case series and was conducted at a single center, spanning from January 2020 to June 2022. Independent of the location and histopathology all cases where intraoperative AR was used were included, accordingly. All patients underwent elective craniotomy with the assistance of AR-guided neuronavigation at the Hospital Universitario Fundación Santa Fe de Bogotá, Bogotá, Colombia. Institutional Review Board approval was obtained. This study was performed in compliance with the Declaration of Helsinki and was done according to our institutional ethics committee approval.
Neuroimaging acquisition protocol
Images were acquired with a 3T scanner (SIGNATM Voyager, GE, GE HealthCare, USA). A 3D BRAVO sequence was acquired with T1 information. Field of view (FOV) 240 slice thickness, 1 mm isotropic, echo time (TE) 3.02, and repetition time (TR) 7.47. A diffusion tensor imaging (DTI) protocol was also included for intra-axial lesions where tractography was required to delineate the lesion’s position and its anatomical relationships: B1000, directions between 40 and 68, FOV 260 mm, Thickness of 3 mm, TR 5585, and TE 76.9. All imaging data were processed using the Elements neuronavigation planning system (Brainlab®, Munich, Germany).
Surgical implementation of AR
During the surgical procedure, patients were placed under general anesthesia and secured using a three-point skull clamp. Optical neuronavigation registration was performed to ensure precise alignment of pre-operative imaging with the surgical site. Subsequently, the surgical microscope (Robotic Visualization System® – KINEVO 900®, Zeiss) was synchronized and integrated with the neuronavigation system to confirm accuracy. The processed images were then imported into AR software, enabling the deployment of both imaging and optical augmentation. This process allowed the superimposition of three-dimensional virtual objects onto the surgical field, enhancing the surgeon’s visual perception during the procedure. The extent of resection (EOR) was calculated as follows: Gross-total resection (GTR) (100%), near-total resection (NTR) (>90%), and sub-total resection (≤90%). All attempts to achieve a GTR were made, however, a maximal safe resection was performed using functional boundaries for intra-axial lesions and respecting neurovascular structures as needed. In Figure 1, we present an illustration of the neurosurgical room in which the neuronavigator and AR are used for brain tumor resection.

- Representation of neurosurgery room with the use of augmented reality. The microscope and the neuronavigator are presented in which images previously taken and prepared under the neuronavigation protocol described above for the resection of brain tumors are superimposed. This figure was created with Biorender (www.Biorender.com).
RESULTS
14 procedures were found where AR guidance was used [Table 1]. Illustrative cases are described in Figures 2-5, which describe the pre-operative findings, the use of intraoperative AR, and the immediate post-operative results. The types of lesions treated with AR technology included one cavernous malformation, seven tumors of glial origin, three meningiomas, one schwannoma, and two metastatic tumors. Regarding the anatomical location of the lesions, AR technology was applied in nine intraaxial and five extraaxial lesions. Among these, one lesion was located at the skull base, one was intraorbital, and one was in the petroclival area.
| Case | Age | Gender | Anatomical location | Pathology | WHO grade | EOR | AR role | Surgical Time (hours) | Length of Stay (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | Left frontal | GBM, IDH -Wild Type | 4 | NTR | Identification of Broca’s area and arcuate fasciculus | 8 | 8 |
| 2 | 52 | F | Left petroclival | Meningioma | 1 | GTR | Identification of the petrous apex and performing an anterior petrosectomy | 7 | 7 |
| 3 | 41 | F | Right frontal | Astrocytoma, IDH-Mutant | III | GTR | To identify the proximity to the motor area | 5 | 5 |
| 4 | 40 | M | Right orbit with intracranial extension to the right cavernous Sinus | Schwannoma | - | NTR | To delimit the intracavernous carotid | 8 | 4 |
| 5 | 20 | M | Left frontal | Astrocytoma, IDH-Mutant | II | GTR | To identify the motor area and monitor the arcuate fasciculus | 5 | 2 |
| 6 | 11 | M | Left temporal (Wernicke’s Area) | GBM, IDH -Wild Type | II | GTR | To identify the Wernicke’s area | 5 | 8 |
| 7 | 43 | F | Anterior fossa | Meningioma | II | GTR | Verify the relationship to the optic nerves | 4 | 5 |
| 8 | 56 | F | Right frontal | Cavernous Malformation | - | GTR | Identification of the drainage vein and the deep afferent artery | 2 | 10 |
| 9 | 57 | F | Left sphenoid wing | Meningioma | I | GTR | Identification of neurovascular structures | 5 | 10 |
| 10 | 59 | F | Left frontal | GBM, IDH -Wild Type | IV | NTR | Identify neurovascular structures, the arcuate fasciculus, and motor area. | 5 | 8 |
| 11 | 79 | F | Left frontal | GBM, IDH -Wild Type | IV | NTR | Relation to vascular structures, arcuate fasciculus, and motor area. | 6 | 13 |
| 12 | 49 | M | Right Frontal | Metastatic Carcinoma, Primary Infiltrating Non-Small Cell Carcinoma | - | NTR | To identify the motor area | 7 | 8 |
| 13 | 54 | M | Bifrontal with extension to the genu of the corpus callosum | Astrocytoma, IDH-Mutant | IV | GTR | To identify the relationship to the anterior cerebral artery | 5 | 6 |
| 14 | 70 | F | Sellar, and parasellar, with extension to the pterygopalatine and infratemporal fossae. | Metastatic Adenocarcinoma, Primary Salivary Cell Adenocarcinoma | - | NTR | To identify the relation to the optic and oculomotor nerves | 9 | 7 |
WHO: World Health Organization, EOR: Extent of resection, GTR: Gross-total resection, NTR: Near-total resection, AR: Augmented reality, GBM: Glioblastoma, IDH: Isocitrate dehydrogenase.
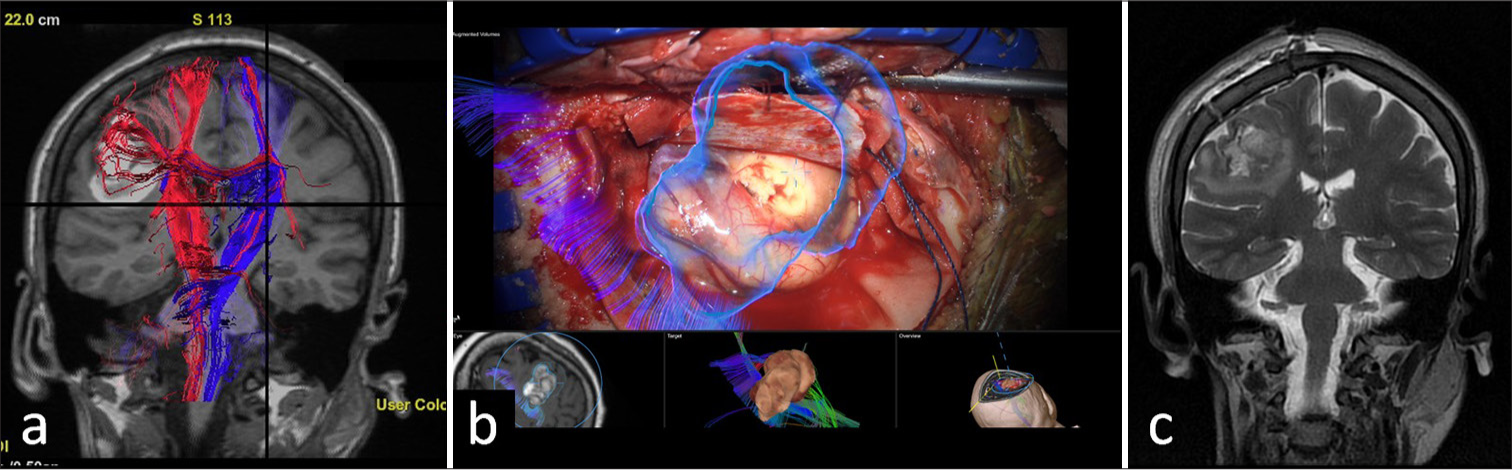
- Frontal perirolandic cavernous malformation. (a) Pre-operative tractography shows a medial displacement and involvement of some lateral fibers of the right corticospinal tract, adjacent to a bleeding site. (b) The cavernous malformation (blue) and the corticospinal tract fibers are superimposed in a microscopic intraoperative image. (c) Immediate post-operative changes of malformation resection in the right precentral region, with hematic debris and the presence of edema around the surgical site are observed.
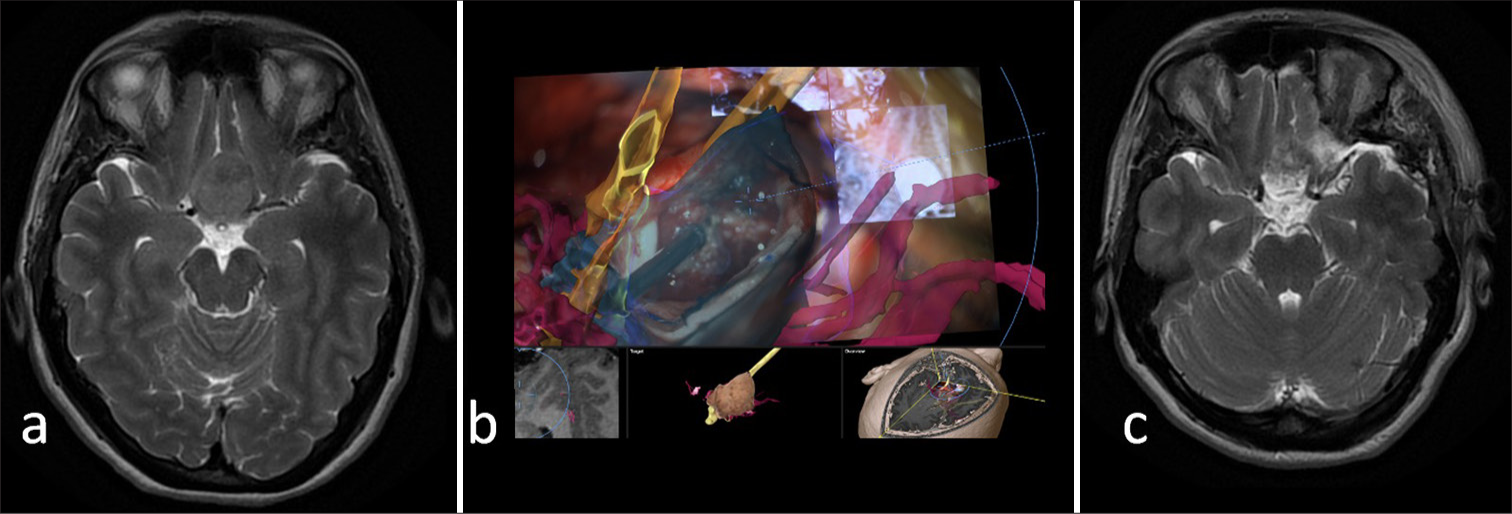
- Tuberculum sellae meningioma. (a) Pre-operative T2 imaging of a meningioma in close relationship with the A1 segment of the anterior cerebral arteries and the anterior aspect of the optic chiasm. (b) Intraoperative microscopic views demonstrating a close relationship of the tumor with the optic nerves and the chiasm (yellow), and the internal carotid and anterior cerebral arteries (red). (c) Post-operative magnetic resonance imaging demonstrating a gross total resection of the tumor.
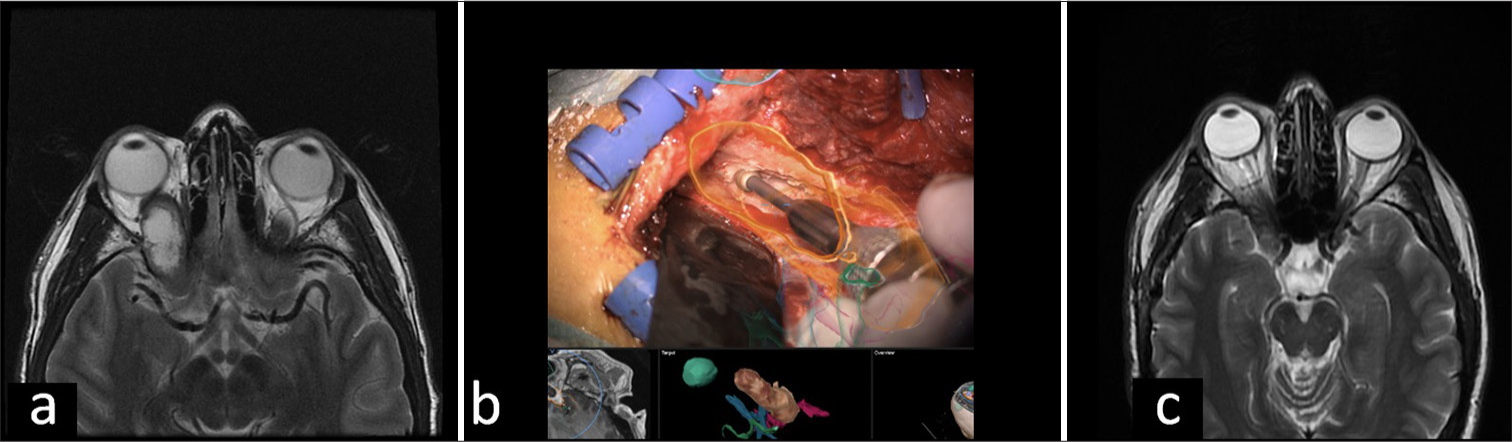
- Anterior skull base schwannoma. (a) An expanding orbital mass with intracranial extension into the right cavernous sinus through the superior sphenoidal fissure is observed in the pre-operative magnetic resonance imaging (MRI). (b) Intraoperative microscopic image demonstrates orbital roof drilling guided by AR while observing the superimposed image of the tumor (orange). (c) Post-operative MRI shows changes in the right pterional craniotomy showing resection of the intra-orbital component of the tumor with a residual in the right cavernous sinus, with minimally extension into the orbital apex.
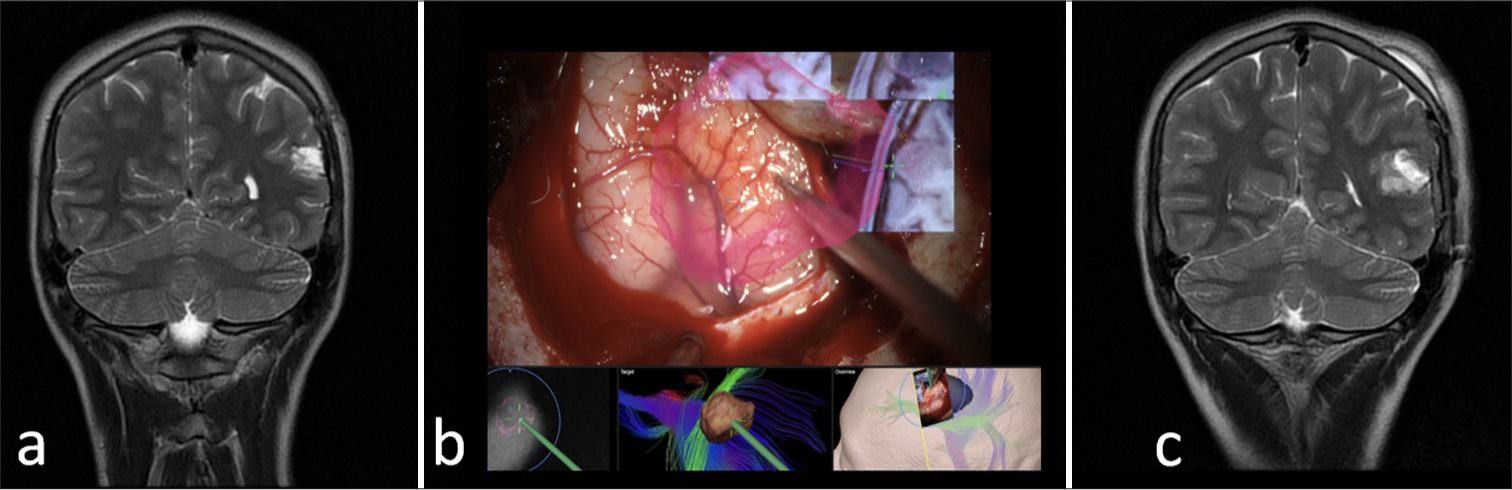
- Parietal glioma. (a) Pre-operative magnetic resonance imaging (MRI) demonstrates a left parietal intra-axial tumor. (b) Intraoperative microscopic view demonstrating in red a superimposed image of the tumor before the cortical stimulation and further corticectomy. (c) Post-operative MRI showing a gross-total resection of the tumor with post-operative edema.
The EOR was either GTR or NTR in all cases. Specifically, six patients underwent NTR, while eight patients achieved a GTR [Table 1]. The patient demographics included eight females and six males, with a median age of 69.5 years. The average surgical time for the procedures was 7 h. No intra- or post-operative complications were observed. The average length of hospital stay was 7 days. The indications for deciding whether to use or not AR navigation were (1) identifying safe boundaries for resection locating arteries and cranial nerves for skull base (5 cases) or intra axial lesions (2 cases) and (2) identifying cortical and subcortical motor and language areas for intra axial lesions (7 cases).
DISCUSSION
The role of AR in neurosurgery
In the medical field, AR has shown significant potential, especially in neurosurgery, enhancing both clinical outcomes and training efficiency.[9] Neurosurgery, which relies heavily on radiological imaging, has integrated AR-based neuronavigation to improve practice and education.[10] Conventionally, neurosurgeons had to look away from the surgical field to consult screens, but AR now allows direct visualization of 3D anatomical details within the surgeon’s view, facilitating more accurate procedures.[11] This real-time overlay of virtual and physical realms enhances the surgical experience.[12,13]
AR significantly enhances surgical landmark identification, navigation, and overall surgeon experience, improving precision and reducing operation time.[12] However, at the beginning of its use, operative room (OR) time, especially before surgical incision, may increase, given the need to prepare both the navigation system and the microscope for adequate imaging injection, as reported in our series. Intraoperative AR fiber tractography has become a crucial tool for planning surgeries in challenging areas like the motor and language areas, helping to avoid post-operative deficits and manage tumors in highly eloquent regions.[14,15] Despite operational improvements in AR-assisted transsphenoidal surgeries, further research is needed to better understand patient outcomes, including mortality, morbidity, and cost-effectiveness.[16] AR technology has notably benefited neuro-oncological procedures, such as gliomas and meningiomas management, offering real-time, updated 3D models superimposed on the surgical field, integrating pre-operative MRI or CT data with real-time anatomy to enhance precision during complex surgeries.[8] Reviews indicate that AR systems improve complex anatomy visualization and intraoperative lesion localization, reducing skin incision size and craniotomy extent. However, challenges like registration accuracy and real-time model updates due to brain shift remain.[9,17,18] In our perspective, the implication of fancy images may not impact improving surgical times or accurate surgeries necessarily. The use of these images may be restricted to specific points like resection of tumor borders only. Likely, the next improvement for this technology may be to have automated processes for imaging injection.
Gliomas
Gliomas, constituting 78% of primary malignant brain tumors, originate from glial progenitor cells such as astrocytes or oligodendrocytes.[14] Maximizing the EOR is crucial for improving survival and the efficacy of adjuvant therapies.[18,19] However, achieving a GTR is challenging due to tumor infiltration into brain parenchyma and functional areas, often causing significant neurological morbidity and mortality.[14,19] Iseki et al. pioneered the evaluation of AR for tumor surgery, finding that using open MRI with real-time navigation significantly increased the EOR in these cases, improving intraoperative image accuracy and aiding tumor removal.[20] The integration of AR with DTI-based high-definition fiber tractography and sodium fluorescein in surgeries for high-grade gliomas markedly improved the EOR and progression-free survival, without increasing complication rates when compared to conventional techniques.[21] Pushing boundaries for improving oncological outcomes is always welcome, and the development of new technologies including AR is promising.
This technology enhances the surgeon’s three-dimensional understanding of the spatial location of fiber tracts, crucial for tumors in the primary motor area where the risk of post-operative motor deficits is high.[21] Integrating AR with essential electrical stimulation mapping overcomes limitations in spatial resolution and fiber tract morphology, offering continuous anatomical-functional feedback during surgery.[22] Studies show that AR combined with intraoperative MRI in these cases results in a significantly higher rate of complete resections and helps preserve neurological functions such as motor skills, visual fields, and language abilities.[19] It is important to remark that AR nor the neuronavigation system can replace the gold standard of intraoperative cortical and subcortical stimulation. Research involving 74 subjects showed that using AR projections with volumetric CT/MRI data reduced intensive care and overall hospital stays by 40% to 50% without extending the surgical duration or increasing complication risks.[23] In our practice, AR has proven beneficial for glioma resections by enabling precise identification and preservation of critical brain structures, such as Broca’s area, arcuate fasciculus, and motor areas including the corticospinal tract, optimizing the EOR while protecting vital neurological functions.
Meningiomas
AR is especially beneficial in meningioma surgeries due to the tumors’ proximity to vital neurovascular structures and bone landmarks.[5] Using pre-operative 3D reconstructions, AR integrates these images into the surgical field through head-up displays (HUDs) and microscopes with integrated HUDs.[24] This technology provides clear visualization of venous structures at both surface and deeper levels, which is particularly beneficial during the excision of extensive meningiomas located in the convexity, along the parasagittal plane, and near the falx cerebri.[25] When it is necessary to incise the dura, AR offers a clear view of the venous sinuses below, which is advantageous for treating falcine meningiomas, allowing for virtual visualization and preservation of the sagittal sinus.[13,25] AR aids in visualizing tumors within the folds of a cerebral sulcus, guiding the surgeon on which sulcus to open. During corticectomy and tumor removal, AR allows the surgeon to view essential vascular and neural structures.[17]
Skull base meningiomas present unique challenges due to their location and complexity. However, the integration of AR with neuronavigation can enhance orientation, reduce adverse events, and potentially increase the EOR.[26] In our experience, AR has been particularly beneficial in complex areas like the petroclival region, enabling precise identification of the petrous apex (and its neurovascular relationships) and facilitating anterior petrosectomy. AR also improved safety by identifying the optic nerves in anterior skull base meningiomas, preventing damage to visual pathways, and accurately delineating the intracavernous carotid artery in meningiomas extending into the cavernous sinus. In addition, AR-enhanced orientation and precision in sphenoid wing meningiomas.
Other pathologies
In our study, we found AR beneficial beyond meningiomas and gliomas. For a cavernous malformation in the right frontal lobe, AR allowed a precise identification of the drainage vein and deep efferent artery, avoiding vascular complications. In such unusual instances, acquiring MRI data immediately following endovascular treatment allows for updated navigation information to be visualized, which can reduce alignment errors, streamline workflow, and improve patient safety and treatment quality.[12] In schwannomas extending to the right cavernous sinus, AR facilitated delimitation of the intra-cavernous carotid, enhancing surgical safety and accuracy. AR was also useful for metastatic tumors, such as those in the right temporal extra-axial region, by identifying relations to the optic and oculomotor nerves. In addition, AR minimized post-operative motor deficits in metastatic carcinoma of the right frontal lobe by accurately identifying the motor area.
Limitations of AR in neurosurgery
In its early years, the largest limitation of AR technology was the hardware itself, but it has dramatically improved recently.[7,27] A significant limitation of neuronavigation is that it does not account for intraoperative brain tissue shift due to positioning changes or cerebrospinal fluid leakage, leading to increased AR alignment errors.[11,28,29] Brain shift, a complex spatiotemporal phenomenon caused by various physiological, chemical, and physical factors, reduces the effectiveness of using pre-operative images for intraoperative surgical guidance.[30] AR technology shares this limitation, necessitating neurosurgeons to consider this inaccuracy, particularly in cortical lesions. However, skull base lesions allow for greater precision due to anatomical bone repairs. Implementing AR requires significant training for the surgical team.[31] Initially, the AR setup added 45 min to conventional navigation times, but recent advancements have reduced this to an average of 12 min. Innovations like live registration updates through intraoperative 3D ultrasound are enhancing AR displays in tumor surgeries, correcting for brain shifts in near-real time.[32]
Successful AR implementation in neurosurgery relies on comprehensive training and participation of the entire surgical team, strategic organization of the surgical room, optimal navigation camera placement, and continuous biomedical engineering support.[30] While initial adoption may increase pre-surgical preparation times, proper coordination and training can reduce these times and improve surgical efficiency.[33] Future integration of ultrasound updates into AR displays for tumor surgeries holds promise, but further research is essential to evaluate AR’s impact on clinical outcomes, resection times, and complication rates. Continuous innovation and rigorous clinical studies are crucial to realizing AR’s full potential in neurosurgery.
Limitations of the study
Despite promising results, our study has several limitations. The small sample size of 14 cases limits the generalizability of the findings, and the single-center design may reduce external validity due to specific practices and technologies unique to our institution. However, this limitation is secondary to a multifactorial barrier. Our institution is located in a middle-income country. The costs related to this technology are enormous in a middle-income economy. Consequently, the approval from healthcare insurance is limited. Even though, we find our study beneficial for LMICs, where the acquisition of this technology may help future neurosurgery teaching. As a retrospective study, it is subject to biases, and the absence of a control group hinders direct comparison with traditional methods. Technological challenges, such as brain shift and registration inaccuracies, can affect AR-guided neuronavigation precision. The learning curve for AR integration may initially prolong surgery times. The follow-up period was limited to the immediate post-operative phase, preventing assessment of long-term outcomes. Future research should involve larger, multi-center studies with control groups and longer follow-up periods to address these limitations and further validate AR’s efficacy in neurosurgery.
CONCLUSION
Our study adds information on AR’s potential to improve surgical accuracy and patient safety. In glioma cases, AR facilitates the precise identification of eloquent areas, crucial for preserving essential functions. For meningiomas and schwannomas, AR improved the accuracy and safety of resections by delineating critical neurovascular structures. Future research should evaluate AR’s impact on clinical outcomes and continue innovation to maximize its benefits in neurosurgical practice.
Ethical approval
The research/study was approved by the Institutional Review Board at Corporate Research Ethics Committee of the Fundación Santa Fe de Bogotá, Colombia., number CCEI-14392-2022, dated July 11, 20222.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Introduction. Virtual and augmented reality in neurosurgery: A timeline. Neurosurg Focus. 2021;51:E1.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality in neurosurgery, state of art and future projections. A systematic review. Front Surg. 2022;9:864792.
- [CrossRef] [PubMed] [Google Scholar]
- Current applications of VR/AR (virtual reality/augmented reality) in pediatric neurosurgery. Adv Tech Stand Neurosurg. 2024;49:19-34.
- [CrossRef] [PubMed] [Google Scholar]
- The virtual vision of neurosurgery: How augmented reality and virtual reality are transforming the neurosurgical operating room. World Neurosurg. 2022;168:190-201.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality for intracranial meningioma resection: A mini-review. Front Neurol. 2023;14:1269014.
- [CrossRef] [PubMed] [Google Scholar]
- An augmented reality system characterization of placement accuracy in neurosurgery. J Clin Neurosci. 2020;72:392-6.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality for enhancing image-guided neurosurgery: Superimposing the future onto the present. World Neurosurg. 2022;157:235-6.
- [CrossRef] [PubMed] [Google Scholar]
- Brain shift in neuronavigation of brain tumors: A review. Med Image Anal. 2017;35:403-20.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality in neurosurgery: A review of current concepts and emerging applications. Can J Neurol Sci. 2017;44:235-45.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality in neurosurgery: A systematic review. Neurosurg Rev. 2017;40:537-48.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality-assisted microsurgical resection of brain arteriovenous malformations: Illustrative case. J Neurosurg Case Lessons. 2022;3:CASE21135.
- [CrossRef] [PubMed] [Google Scholar]
- Dex-ray: Augmented reality neurosurgical navigation with a handheld video probe. Neurosurgery. 2009;65:795-807.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative augmented reality fiber tractography for primary motor area glioma resection. World Neurosurg. 2023;180:111.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative augmented reality fiber tractography complements cortical and subcortical mapping. World Neurosurg X. 2023;20:100226.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality-assisted skull base surgery. Neurochirurgie. 2014;60:304-6.
- [CrossRef] [PubMed] [Google Scholar]
- Preliminary study on the clinical application of augmented reality neuronavigation. J Neurol Surg A Cent Eur Neurosurg. 2013;74:71-6.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-operative applications of augmented reality in glioma surgery: A systematic review. Front Surg. 2023;10:1245851.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of virtual and augmented reality based on intraoperative magnetic resonance imaging and functional neuronavigation in glioma surgery involving eloquent areas. World Neurosurg. 2016;96:375-82.
- [CrossRef] [PubMed] [Google Scholar]
- Intelligent operating theater using intraoperative open-MRI. Magn Reson Med Sci. 2005;4:129-36.
- [CrossRef] [PubMed] [Google Scholar]
- Microscope-based augmented reality with diffusion tensor imaging and fluorescein in insular glioma resection. Neurosurg Focus Video. 2022;6:V10.
- [CrossRef] [PubMed] [Google Scholar]
- Simulation of surgery for supratentorial gliomas in virtual reality using a 3D volume rendering technique: A poor man's neuronavigation. Neurosurg Focus. 2021;51:E23.
- [CrossRef] [PubMed] [Google Scholar]
- Use of a volumetric target for image-guided surgery. Neurosurgery. 2006;59:651-8.
- [CrossRef] [PubMed] [Google Scholar]
- Navigation-linked heads-up display in intracranial surgery: Early experience. Oper Neurosurg (Hagerstown). 2018;15:184-93.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality neurosurgical planning and navigation for surgical excision of parasagittal, falcine and convexity meningiomas. Br J Neurosurg. 2010;24:69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Microscope-based augmented reality with intraoperative computed tomography-based navigation for resection of skull base meningiomas in consecutive series of 39 patients. Cancers (Basel). 2022;14:2302.
- [CrossRef] [PubMed] [Google Scholar]
- Augmented reality in neurosurgery. Arch Med Sci. 2018;14:572-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical experience and perception in stereo augmented reality surgical navigation. Lecture notes in computer science (including subseries lecture notes in artificial intelligence and lecture notes in bioinformatics) Lect Notes Comput Sci. 2004;3150:369-76.
- [CrossRef] [Google Scholar]
- Augmented reality visualization system for intravascular neurosurgery. Comput Aided Surg. 1998;3:239-47.
- [CrossRef] [Google Scholar]
- Presurgical and intraoperative augmented reality in neuro-oncologic surgery: Clinical experiences and limitations. World Neurosurg. 2019;128:268-76.
- [CrossRef] [PubMed] [Google Scholar]
- Advantages and challenges associated with augmented reality for education: A systematic review of the literature. Educ Res Rev. 2017;20:1-11.
- [CrossRef] [Google Scholar]
- Intra-operative correction of brain-shift. Acta Neurochir (Wien). 2014;156:1301-10.
- [CrossRef] [PubMed] [Google Scholar]
- Improving patient specific neurosurgical models with intraoperative ultrasound and augmented reality visualizations in a neuronavigation environment. Lecture notes in computer science (including subseries lecture notes in artificial intelligence and lecture notes in bioinformatics). Vol. 9401 In: In: Conference: Workshop on Clinical Image-Based Procedures. 2016. p. :28-35.
- [CrossRef] [Google Scholar]







