Translate this page into:
Expression and association of vascular endothelial growth factor, vascular endothelial growth factor receptor, and phosphorylated signal transducer and activator of transcription factor 3 in malignant gliomas
*Corresponding author: Bheemanathi Hanuman Srinivas, Department of Pathology, Jawaharlal Institute of Post-graduate Medical Education and Research, Puducherry, India. srinivas.bh08@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Edura P, Vokuda R, Ramamoorthi S, Srinivas BH, Verma SK, Sasidharan G. Expression and association of vascular endothelial growth factor, vascular endothelial growth factor receptor, and phosphorylated signal transducer and activator of transcription factor 3 in malignant gliomas. J Neurosci Rural Pract 2023;14:723-8.
Abstract
Objectives:
Angiogenesis is one of the main characteristic features of malignant gliomas. Phosphorylated signal transducer and activator of transcription factor 3 (pSTAT3) is not only involved in glioma cell proliferation, anti-apoptosis, and immunosuppression but also plays a key role in cell migration and invasion. Constitutively, activated pSTAT3 induces expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR, leading to endothelial cell proliferation and abnormal microvascular formation causing peritumoral edema (PTE). PTE is one of the significant contributors to mortality in malignant gliomas. Therefore, understanding the molecular mechanism involved in the evolution of gliomas is necessary. This study was to assess the level of expression of pSTAT3, VEGF, and VEGFR in malignant gliomas and analyze the extent of PTE and the extent of expression of one or more of these markers.
Materials and Methods:
This study included 84 patients categorized as per the World Health Organization classification of central nervous system tumors into grade IV, III, and II gliomas to investigate the expression of pSTAT3, VEGF, and VEGFR by immunohistochemistry. Furthermore, the presence or absence of PTE was determined using magnetic resonance imaging/computed tomography scans in these patients.
Results:
The association between the markers (pSTAT3, VEGFR, and VEGF) and the extent of PTE in these patients was statistically significant (P < 0.05).
Conclusion:
The pSTAT3, VEGF-R, and VEGF signaling pathways could contribute to peritumoral edema and might be a regulatory mechanism during PTE formation during tumorigenesis and progression.
Keywords
Malignant gliomas
Phosphorylated signal transducer and activator of transcription factor 3
Vascular endothelial growth factor
Vascular endothelial growth factor receptor
INTRODUCTION
Gliomas are the most common brain tumors, attributing to 80% of cases and 30% of malignant tumors. According to the World Health Organization (WHO) classification, there are four grades; grade I is predominantly benign, and grades II–IV are malignant gliomas.[1] Lower-grade gliomas (WHO grade I) have a better prognosis than higher-grade gliomas (II, III, and IV). Of these, glioblastoma (GBM) is the most common and most lethal. Primary GBM arises de novo, while secondary GBM arises from the lower-grade gliomas. Primary and secondary GBMs cannot be differentiated histologically but differ in their genetic and epigenetic profiles.[2] Magnetic resonance imaging (MRI) helps visualize changes in the morphological characters and directly reflects biochemical changes in the tumor itself and surrounding tissue. Despite aggressive treatment, the median survival rate in these patients is <15 months.[3] The major challenge in treating gliomas is the extent of surgical resection and resistance to chemoradiotherapy. Since gliomas have a distinctive character of infiltrating adjacent brain parenchyma, thus compromising complete surgical removal of the tumor. Despite the incurability and resistance to chemoradiotherapy, the identification of proper drivers of infiltration of gliomas is needed for anti-invasive therapy. The recent identification of the signal transducer and activator of the transcription factor 3 (STAT3) pathway, a molecular hub for signal transduction in gliomas, may be considered as potential therapeutic target.[4] STAT3 is generally absent in normal brain tissue. However, it gets activated by growth factor receptors at the tyrosine residues and gets phosphorylated, leading to altered normal cell functions. Janus Kinases (JAK1 and JAK2) are the primary upstream mediators of STAT3 activation.[5] STAT3 is involved in glioma cell proliferation and anti-apoptotic activity, aiding in invasion and infiltration. STAT3 also regulates vascular endothelial growth factor (VEGF), resulting in neoangiogenesis,[6] a prerequisite for tumor growth. Hypoxia stimulates VEGF and gets upregulated by various oncogenes and proto-oncogenes.[7] Peritumoral edema (PTE) is the characteristic feature of malignant gliomas. VEGF plays a pivotal role in the PTE of gliomas by down-regulating the tight junction proteins like occludin, leading to the formation of cleft and fenestra between the endothelial cells, leading to increased vascular permeability.[8] PTE is an important focus, where tumor cells migrate to the adjacent normal brain parenchyma creating a chance of recurrence.[9] At diagnosis, these tumors show heterogeneous contrast enhancement with PTE on MRI. Increased mortality is reported in these patients due to PTE, indicating a poor prognosis. To address this issue, we analyzed the extent of PTE and its association with phosphorylated signal transducer and activator of transcription factor 3 (pSTAT3), VEGF, and VEGFR expression in malignant gliomas.
MATERIALS AND METHODS
This retrospective study was conducted at Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, a tertiary care center. The study is conducted after clearance from the Ethics Committee of the institute.
Eighty-four patients were operated on for tumor resection in the Department of Neurosurgery and diagnosed as gliomas in the Department of Pathology, JIPMER, fulfilling the WHO criteria/grade for astrocytoma/oligodendroglioma/ GBM grades II, III, and IV were included in the study. Grade l gliomas and patients who received neoadjuvant chemotherapy, recurrent cases, and ependymomas were excluded from the study.
Clinical and radiological examination details were obtained from patient record archives. Pre-operative MRI/computed tomography (CT) data were collected for every patient. All MRI scans were evaluated by a radiologist who was blinded to pathological diagnosis. A region of very bright T2-W signal and low T1-W signal without enhancement around the tumor was determined as PTE.
Grading of the extent of PTE was measured using MRI scans as explained by Carlson et al.[10]
Grade 1 – Maximum distance between the edema’s outer edge and the nearest tumor margin point <2 cm
Grade 2 – Edema extending ≥2 cm from the tumor margin in axial T2-W images.
In the event of the unavailability of an MRI scan, the hypodensity seen around the tumor in a CT scan was used to grade the edema.
Edema shape was classified as follows:
Roundish – Shape is regular/round, and
Irregular – Shape tends to be irregular such as radial or finger-like, as shown in [Figure 1].
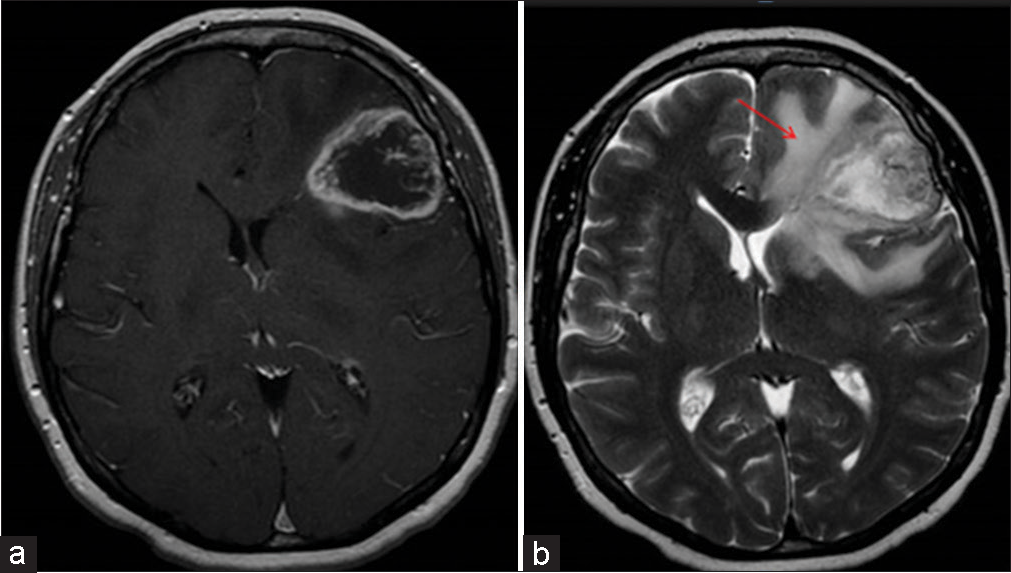
- Contrast-enhanced magnetic resonance imaging shows a tumor with perilesional edema (a) well-circumscribed lesion with enhancement representing edema regular in shape, size <2 cm. (b) tumor with necrosis and edema irregular in shape with >2 cm in size (indicated by arrow).
Immunohistochemistry (IHC)
IHC was performed on the freshly cut formalin fixed paraffin embedded tissue (4 µm). The following primary antibodies were used pSTAT3 (phospho Y705) rabbit monoclonal antibody (1:100 dilution) Abcam; VEGFR (ab39256) rabbit monoclonal antibody, (1:200 dilution) Abcam; and VEGF rabbit monoclonal antibody (1:200 dilution), thermo scientific. Normal kidney and colon adenocarcinoma were used as external control and endothelial cell nucleus was taken as internal control for pSTAT3, whereas breast and placenta tissue were positive controls for VEGFR and VEGF, respectively. In addition, endothelial cell cytoplasm was taken as an internal control for VEGFR and VEGF.
The semi-quantitative scoring system used for grading the expression of pSTAT3, VEGF, and VEGFR is as follows:
0: Negative; +1: < 20% of positive cells (weak); +2: 20–50% of positive cells (Moderate); +3: >50% of positive cells (strong)
Statistical analysis
The resultant data were analyzed using SPSS 19.0 statistical software. The distribution of categorical variables such as gender, peritumoral edema, and tumor grade was expressed in frequency percentage. The distribution of continuous variables such as age and expression of VEGF, VEGFR, and pSTAT3 were represented in terms of mean with standard deviation. The comparison of the above-stated continuous variables with PTE and tumor grade was made using Fisher’s exact test. The relationship between IHC markers and PTE was carried out using correlation analysis. The independent factors associated with the level of IHC markers were carried out using multiple linear regression analysis. All statistical analyses were carried out at a 5% significance level with P < 0.05 considered statistically significant.
RESULTS
In the present study, 84 patients were enrolled per inclusion and exclusion criteria. These included 51 cases of Grade IV (GBMs), 16 cases of Grade III (anaplastic astrocytoma and oligodendroglioma), and 14 cases of Grade II (astrocytoma and oligodendroglioma). Of the included 84 patients, 52 were male and 32 were female, with an M: F ratio equal to 1.6:1. Mean age of the patient was 41.3 ± 13.3 years. Most patients presented with symptoms of seizures 32 (38%) as a chief complaint, followed by altered sensorium 18 (21%), diplopia, and slurred speech. Headache and vomiting indicated increased intracranial tension in 11 (13.09%) patients.
Peritumoral edema was reported in 63/84 (75%) patients. Of these, PTE was grade 1 (<2 cm) in 33 (52.3%) and grade 2 (≥2 cm) in 30 (47.6%) patients. The edema was round or regular in shape in 34 (53.9%) and irregular or finger-like in 29 (46.0%) cases.
The study demonstrated a positive association of immunohistochemical expression of VEGF, VEGFR, and pSTAT3 across the various grades of diffuse gliomas [Figure 2]. The same is shown in [Tables 1-3]. Significant correlations in the expression of pSTAT3, VEGF, and VEGFR were seen in GBM, anaplastic astrocytoma, and anaplastic oligodendroglioma, while, in diffuse astrocytoma and oligodendrogliomas, this correlation was insignificant. The positive expression of the above markers was also significantly associated with the presence of PTE, along with its grades (P < 0.05). The expression and corelation are represented in [Table 4].
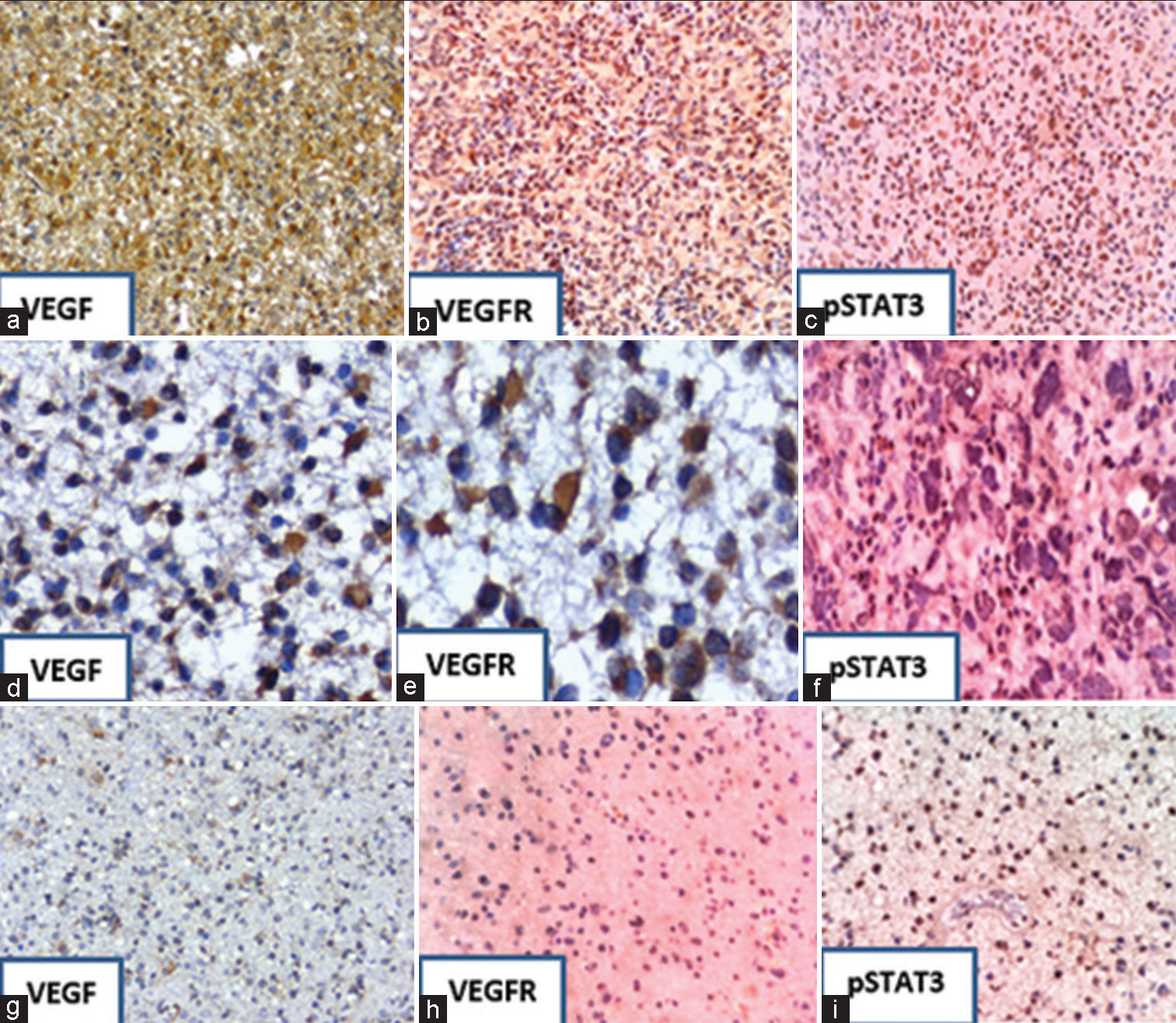
- Immunohistochemical staining showing the expression of VEGF, VEFGR, and pSTAT3; Strong expression (a-c) in GBM (Grade IV) and from (d-f) moderate expression in anaplastic astrocytoma (Grade III). From (g-i), there is a weak expression of markers in diffuse astrocytoma (Grade II). Immunostaining is also seen in endothelial cells (f and i). Magnification ×200. VEGF: Vascular endothelial growth factor, VEGFR: Vascular endothelial growth factor receptor, and pSTAT3: Phosphorylated signal transducer and activator of transcription factor 3.
| Diagnosis | Negative (%) | Weak (<10% of tumor cells positive) (%) | Moderate (10–50% of tumor cells positive) (%) | Strong (>50% of tumor cells positive) (%) | Total | P-value |
|---|---|---|---|---|---|---|
| Grade II | 2 (11.5) | 4 (23.5) | 6 (35.2) | 5 (29.4) | 17 | <0.02 |
| Grade III | 2 (12.5) | 4 (25.5) | 4 (25) | 6 (37.5) | 16 | |
| Grade IV | 1 (1.9) | 4 (7.8) | 23 (45) | 23 (45) | 51 | |
| Total | 5 (5.9) | 12 (14.2) | 33 (39.2) | 34 (40.4) | 84 |
VEGF: Vascular endothelial growth factor
| Diagnosis | Negative (%) | Weak (<10% of tumor cells positive) (%) | Moderate (10–50% of tumor cells positive) (%) | Strong (>50% of tumor cells positive) (%) | Total | P-value |
|---|---|---|---|---|---|---|
| Grade II | 5 (29.4) | 2 (11.7) | 4 (23.5) | 6 (35.2) | 17 | <0.03 |
| Grade III | 0 (0) | 1 (6.2) | 5 (31.2) | 11 (68.7) | 16 | |
| Grade IV | 1 (1.9) | 8 (15.6) | 18 (35) | 24 (47) | 51 | |
| Total | 6 (7.1) | 11 (13.09) | 27 (32.1) | 41 (48.8) | 84 |
VEGFR: Vascular endothelial growth factor receptor
| Diagnosis | Negative (%) | Weak (<10% of tumor cells positive) (%) | Moderate (10–50% of tumor cells positive) (%) | Strong (>50% of tumor cells positive) (%) | Total | P-value |
|---|---|---|---|---|---|---|
| Grade II | 9 (52.9) | 2 (11.7) | 3 (17.6) | 3 (17.6) | 17 | <0.02 |
| Grade III | 2 (12.5) | 2 (12.5) | 6 (37.5) | 6 (37.5) | 16 | |
| Grade IV | 2 (3.9) | 14 (27.4) | 17 (33.3) | 18 (35.4) | 51 | |
| Total | 12 (14.2) | 18 (21.4) | 26 (30.9) | 27 (32.1) | 84 |
pSTAT3: phosphorylated signal transducer and activator of transcription factor 3
| S. No. | IHC marker | No PTE n=21 (%) | PTE Grade 1 (<2 cm) n=33 (%) | PTE Grade 2 (>2 cm) n=30 (%) | P<0.05 | PTE shape regular n=34 (%) | PTE shape irregular n=29 (%) | P<0.05 |
|---|---|---|---|---|---|---|---|---|
| 1. | VEGF | |||||||
| 0 | 8/21 (66.) | 2/33 (6.0) | 0/30 (0) | <0.02 | 2/34 (5.8) | 0/29 (0) | 0.159 | |
| 1 | 4/21 (33) | 2/33 (6.0) | 5/30 (16.7) | 2/34 (5.8) | 5/29 (17.2) | |||
| 2 | 6/21 (50) | 17/33 (51) | 9/30 (30) | 13/34 (38.2) | 12/29 (41.3) | |||
| 3 | 3/21 (25) | 12/33 (36.3) | 16/30 (53.3) | 17/34 (50) | 12/29 (41.3) | |||
| 2. | VEGFR | |||||||
| 0 | 5/21 (41.6) | 2/33 (6.06) | 0/30 (0) | <0.01 | 2/34 (5.8) | 0/29 (0) | 0.147 | |
| 1 | 4/21 (33.3) | 4/33 (12.1) | 4/30 (13.3) | 5/34 (14.7) | 3/29 (10.3) | |||
| 2 | 8/21 (66.6) | 16/33 (48.4) | 7/30 (23.3) | 13/34 (38.2) | 10/29 (34.4) | |||
| 3 | 4/21 (33.3) | 11/33 (33.3) | 20/30 (6.7) | 14/34 (41.1) | 16/29 (55.2) | |||
| 3. | pSTAT3 | |||||||
| 0 | 11/21 (52.3) | 1/33 (3.0) | 2/30 (6.6) | <0.02 | 1/34 (2.9) | 2/29 (6.8) | 0.228 | |
| 1 | 5/21 (41.6) | 11/33 (33.3) | 5/30 (16.6) | 10/34 (29.4) | 7/29 (24.1) | |||
| 2 | 3/21 (25) | 15/33 (45) | 8/30 (26.6) | 13/34 (38.2) | 9/29 (31.0) | |||
| 3 | 2/21 (16.6) | 6/33 (18.1) | 15/30 (50) | 10/34 (29.4) | 11/29 (37.9) |
IHC: Immunohistochemistry, PTE: Peritumoral edema, VEGF: Vascular endothelial growth factor, VEGFR: Vascular endothelial growth factor receptor, pSTAT3: phosphorylated signal transducer and activator of transcription factor 3
DISCUSSION
The outcomes associated with gliomas are very poor, despite aggressive treatment. In the present study, we evaluated the expression of pSTAT3, VEGF, and VEGFR across tumor grades and correlated the expression of pSTAT3, VEGF, and VEGFR with PTE. The role of STAT3 is to maintain and proliferate malignant cells and also plays a role in the transition to more malignant subtypes.
A study by Wang et al.,[11] which included 84 cases of GBM, found pSTAT3 positivity in 20% (median) of cases and VEGF expression in 65% (median) of the WHO grade IV gliomas using IHC and found a statistically significant association of these proteins with PTE. In the present study, we detected the expression of pSTAT3, VEGFR, and VEGF, not only in the WHO grade IV but also in the WHO grade III and II tumors. Higher expression of these markers is found in higher grades of tumors.
A retrospective study by Susman et al. was done to evaluate the prognostic role of STAT 3, they reported an increase in pSTAT3 level which is associated with poor prognosis in GBM patients. With a median survival of 8.9 months in patients expressing more than 20% positivity in comparison with 13.7 months in patient s expressing <20% positivity of STAT3. Hence, emphasizing the role of STAT3 as target for treatment.[12] In this study, patient survival was not studied and only the expression of STAT3 with tumor grade and its association with PTE was evaluated.
A study by Leventoux et al. claimed that high-density foci of grade II gliomas showed a high percentage of STAT3-positive cells, which indicated that the STAT3 pathway activated in these cells led to its malignant behavior.[13] A study by Wang et al. reported high expression in VEGF and PI3K levels in glioma cancer stem cells in grades III–IV compared to grade II cases using RT-qPCR.[14] Another study conducted by Carlson et al. proved that VEGF expression indicated poorer survival in patients with extensive edema with a concordance of 95%.[10] In the present study, VEGF expression was significantly associated with PTE, compromising survival. This might be due to the infiltration of tumor cells into the adjacent brain tissue.
Wu et al.[15] observed that PTE and necrosis shown by MRI scans were independent predictors of unfavorable prognosis in GBM (the WHO grade IV) patients. However, another study reported that VEGF-C and -D and their coreceptors VEGFR 2 and VEGFR 3 were overexpressed in the majority of GBMs. However, the IHC expression levels did not correlate with overall survival and isocitrate dehydrogenase status.[16] One study reported that VEGF stimulates GBM stem cells under both normoxic and hypoxic conditions in a dose-dependent manner, while VEGFR1 has a negative feedback effect on VEGFR2.[17]
Huang et al. found that both VEGF and VEGFR expression in various brain tumors differ and are not necessarily parallel. In our study, both are highly expressed in grade IV tumors.[18] Zhao et al. noticed pSTAT3 and VEGFR expression in small-cell lung carcinoma and lymph node metastasis through IHC.[19] Similar effects are also seen in head and neck squamous cell carcinoma, colon, breast, kidney, and endometrial cancers. The current study helps us understand the STAT3-VEGF signaling pathway in gliomas through the JAK-STAT pathway. pSTAT3, VEGF plays a pivotal role in apoptosis, migration, invasion, and neoangiogenesis, which are hallmarks of glioma aggressiveness. Thus, STAT3 knockdown renders future promise for effective chemoradiotherapy. The implication of our study is that many pathways have been reported in the pathogenesis of malignant glioma. STAT3-VEGF signaling pathway is considered as the most important one due to the convergence of several pathways at p-STAT3 and also through modulation of genes implicated in cell proliferation, apoptosis, migration, invasion, and neoangiogenesis which are hallmarks of glioma aggressiveness. Hence, there is the intriguing possibility that p-STAT3 can be considered as a therapeutic target and increase the survival of these patients.
This study has a few limitations. Quantification of the molecular markers was not carried out in these samples. Furthermore, a follow-up survival analysis was not done to investigate the survival period in these patients.
CONCLUSION
This is the first study in the Indian population to confirm that PTE extent is positively associated with pSTAT3, VEGF, and VEGFR expression in high-grade gliomas. Our study provides evidence that the pSTAT3-VEGFVEGFR signaling pathway might play a pivotal role in alleviating the PTE. Therefore, clinical approaches should consider including a STAT3 inhibitor in the therapeutic protocol to improve tumor response to chemotherapy and radiotherapy.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-Assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
- [CrossRef] [PubMed] [Google Scholar]
- The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764-72.
- [CrossRef] [PubMed] [Google Scholar]
- Chemoradiotherapy in malignant glioma: Standard of care and future directions. J Clin Oncol. 2007;25:4127-36.
- [CrossRef] [PubMed] [Google Scholar]
- Signal transducer and activator of transcription-3: A molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675-84.
- [CrossRef] [PubMed] [Google Scholar]
- The role of JAK-STAT signaling within the CNS. JAKSTAT. 2013;2:e22925.
- [CrossRef] [PubMed] [Google Scholar]
- Nuclear translocation of phosphorylated STAT3 regulates VEGF-A-induced lymphatic endothelial cell migration and tube formation. Biochem Biophys Res Commun. 2011;412:441-5.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia, angiogenesis and mechanisms for invasion of malignant gliomas In: Evolution of the Molecular Biology of Brain Tumors and the Therapeutic Implications. United Kingdom: IntechOpen; 2013.
- [CrossRef] [Google Scholar]
- AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. 2008;8:19-26.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging molecular mechanisms of brain tumour oedema. Br J Neurosurg. 2001;15:101-8.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between survival and edema in malignant gliomas: Role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res. 2007;13:2592-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association of pSTAT3-VEGF signaling pathway with peritumoral edema in newly diagnosed glioblastoma: An immunohistochemical study. Int J Clin Exp Pathol. 2014;7:6133-40.
- [Google Scholar]
- The role of p-Stat3 Y705 immunohistochemistry in glioblastoma prognosis. Diagn Pathol. 2019;14:124.
- [CrossRef] [PubMed] [Google Scholar]
- Transformation foci in IDH1-mutated gliomas show STAT3 phosphorylation and downregulate the metabolic enzyme ETNPPL, a negative regulator of glioma growth. Sci Rep. 2020;10:5504.
- [CrossRef] [PubMed] [Google Scholar]
- High expression of VEGF and PI3K in glioma stem cells provides new criteria for the grading of gliomas. Exp Ther Med. 2016;11:571-6.
- [CrossRef] [PubMed] [Google Scholar]
- Peritumoral edema shown by MRI predicts poor clinical outcome in glioblastoma. World J Surg Oncol. 2015;13:97.
- [CrossRef] [PubMed] [Google Scholar]
- The immunohistochemical landscape of the VEGF family and its receptors in glioblastomas. Surg Exp Pathol. 2020;3:9.
- [CrossRef] [Google Scholar]
- VEGF promotes proliferation of human glioblastoma multiforme stem-like cells through VEGF receptor 2. ScientificWorldJournal. 2013;2013:417413.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of VEGF and its receptors in different brain tumors. Neurol Res. 2005;27:371-7.
- [CrossRef] [PubMed] [Google Scholar]
- Expression and clinical significance of STAT3, P-STAT3, and VEGF-C in small cell lung cancer. Asian Pac J Cancer Prev. 2012;13:2873-7.
- [CrossRef] [PubMed] [Google Scholar]







