Translate this page into:
Etiological and Radiological Spectrum of Longitudinal Myelitis: A Hospital-Based Study in North East India
Baiakmenlang Synmon Department of Neurology, North Eastern Indira Gandhi Regional Institute of Health & Medical Sciences (NEIGRIHMS) Shillong India baiakmenlangsynmon@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction An inflammatory lesion of the spinal cord where three or more than three vertebral segments of the cord is involved is called longitudinal extensive myelitis (LETM). It has several varied causes out of which neuromyelitis optica (NMO) and its spectrum disorder have received a distinct entity. Various radiological and clinical features help us to suspect an etiology which then further guides us into the treatment protocol and prognosis of the patients.
Materials and Methods A retrospective study performed in a referral center in North East India in 15 months. Thirty-two patients of LETM were enrolled based on clinical and radiological available data. An attempt was made to classify the various etiologies and correlate with their radiological findings.
Results The most common etiology noted was NMO seen in 7 patients (21.8%) followed by tuberculosis (TB) (18.7%) and post-infection myelitis (18.7%). Other etiology seen was acute disseminated encephalomyelitis (6.24%), spinal cord infarct (3.12%), radiation myelitis (6.24%), Japanese encephalitis sequalae (3.12%), systemic lupus erythematosus (3.12%), and remained undiagnosed in six patients (18.7%). Radiologically, cervico-dorsal spine was most common location in NMO (71%) whereas dorsolumbar in TB (50%). The lesion was predominantly central in both NMO (100%) and TB (80%) as compared with the other causes of LETM. It was noted that more than 50% of the transverse area of the cord was involved in both NMO (71%) and TB (50%), but < 50% involvement were more common in the post-infectious and others causes of LETM.
Conclusion LETM has a various differential diagnosis, infection need to be kept in mind while ruling out NMO. Radiological features can suggest or help differentiate the various etiologies of LETM but NMO and infection like TB almost has the same features except for a different cord site predilection.
Keywords
longitudinal extensive myelitis (LETM)
neuromyelitis optica (NMO)
tuberculosis
Introduction
Myelopathy can refer to any form of spinal cord pathology whereas myelitis refers to an inflammatory or infectious process.1 An inflammatory lesion of the spinal cord that involves a contiguous vertebral segments of three or more than three is called longitudinal extensive myelitis (LETM).2 The clinical course of LETM is characterized by single or multiple attacks of paraparesis or tetraparesis or quadriparesis with or without sensory deficits and bowel/bladder disturbances and sometime respiratory involvement.3 An early distinction both clinically and radiologically among the various etiologies like neuromyelitis optica (NMO), infectious causes, autoimmune causes like systemic lupus erythematosus (SLE) and sarcoid, arteriovenous (AV) fistula, and many more is crucial in providing accurate prognosis and guiding the treatment strategy. It is important to identify NMO from other etiologies because of its increase risk of future relapse, disability, and requirement of an early accurate treatment.4 5 Longitudinal myelitis is common among all age group. Availability of serum aquaporin-4 antibodies (AQpO4) made the diagnosis of NMO easier but differentiating NMO spectrum disorder (NMOSD) from other causes of LETM still poses a challenge needing a clinical and radiological experience. With this in mind we tried analyzing the various etiologies and radiological features of LETM patients who presented to our institute.
Materials and Methods
This study was conducted in a retrospective cross-section manner in a referral center in North East India within the duration of 15 months from December 2018 to February 2020. The clinical and radiological data of the longitudinal myelitis were collected and analyzed.
All the patients with long segment myelitis of more than three vertebral involvements on magnetic resonance imaging (MRI) were included in the study. The inadequate MRI study or images degraded with motion artifact were excluded from the study. MRI spine using 1.5 Tesla (T) strength with sequences like sagittal and axial T1, T2, fluid attenuation inversion recovery, contrast study, short-tau inversion recovery (STIR), diffusion-weighted imaging, and magnetic resonance spectroscopy were done. MRI spine, brain, and optic nerve study was done in all patients. We evaluated the lesion of the spine, its extent and location, both longitudinally and transversely, the lesion was divided as involving more than or less than 50% of the spinal cord cross-sectional area. Lesion distribution was noted weather peripheral or central or both. Presences of cord expansion, brainstem involvement, optic nerve involvement, or brain involvement in various areas were noted. Enhancement of the lesion after contrast was noted whether patchy and heterogeneous or homogeneous and well defined in nature.
An attempt was made to identify the various etiologies among the worked-up cases of LETM. Most of the cases had undergone a detailed neurological workup, routine blood test, and viral markers with serum Venereal Disease Research Laboratory was done in all cases. Serum autoimmune antibody (ANA), thyroid profile, and vitamin B12 analysis was done in all cases. Routine cerebrospinal fluid (CSF) analysis was done in all patients, whereas CSF gene expert for tuberculosis (TB) and viral study analysis was done only in few cases as per clinical indication. Serum AQpO4 and CSF oligoclonal band (OCB) were also done in a few cases as per clinical requirements.
The diagnosis of acute transverse myelitis (ATM) was based on the criteria proposed by the Transverse Myelitis Consortium Working Group.6 Acute disseminated encephalomyelitis (ADEM) diagnosis was made based on the criteria in the pediatric age group.7 Post-infectious myelitis (PIM) was labeled in the presence of clear history suggestive of infection preceding onset of neurological symptoms within 30 days. Revised McDonald criteria was used for the diagnosis of multiple sclerosis (MS).8 NMO was diagnosed using the 2015 diagnostic criteria.9 Infectious myelopathy was diagnosed based evidence of infection by a positive serology or intrathecal specific antibody synthesis or detection of pathogen in CSF and evidence of other organ involvement with its temporal relationship. Paraneoplastic myelopathy or radiation myelitis was labeled in the presence of malignancy or paraneoplastic antibody or history of radiation given with correlation to the myelitis, after exclusion of other causes.
Results
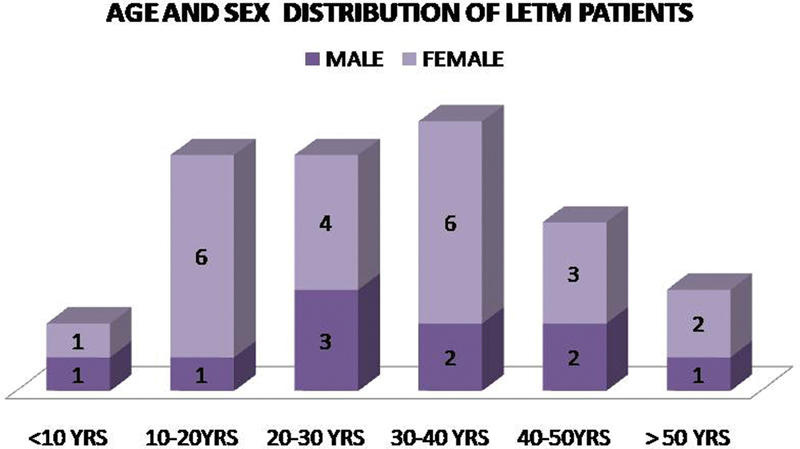
Thirty-two patients of longitudinal myelitis were included in the study for 15 months' duration. Their age ranged from 7 to 64 years and the most common age group seen was between 30 and 40 years. Female (68%) were more predominant in comparison to male (42%) as shown in Fig. 1. The most common clinical presentation was paraparesis with bladder involvement seen in 27 patients (84%). Quadriparesis was seen in 4 patients (13%) and hemiparesis in 1 patient (3.3%). Recurrent paraparesis was seen in 3 patients (9.3%). The duration of presentation was acute < 14 days in 13 patients (40%) and subacute (2–4 weeks) in 10 patients (31%) and chronic (> 4 weeks) in 9 patients (28%).
-
Fig. 1 Age and sex distribution. LETM, longitudinal extensive myelitis.
Fig. 1 Age and sex distribution. LETM, longitudinal extensive myelitis.
An etiological diagnosis was made in 26 patients and remained undiagnosed in 6 patients. In the present series NMO was the most common etiology seen in 7 patients (21.8%) followed by infection with TB as the etiology seen in 6 patients (18.7%) and PIM in 6 patients (18.7%). The other etiology noted was ADEM (6.24%), radiation myelitis (6.24%), spinal cord infarct (3.12%), SLE seen in one patient (3.12%), and Japanese encephalitis (JE) sequalae seen in one patient (3.12%) as shown in Fig. 2.

-
Fig. 2 Etiological spectrum of longitudinal extensive myelitis (LETM) patients.
Fig. 2 Etiological spectrum of longitudinal extensive myelitis (LETM) patients.
The diagnosis of NMO (Supplementary Fig. S1, online only) was made in 7 patients based on clinical and radiological criteria. Positive serum AQpO4 was found in 6 out of 11 patients tested, one patient was however labeled as NMOSD despite a negative test. OCB was positive in 2 out of 4 patients tested; both patients also tested AQpO4 positive and a clinical diagnosis of NMO.
The diagnosis of TB was made by an abnormal CSF study suggestive of an infectious etiology. It was confirmed microbiologically in two patients, one with CSF gene expert positive for the bacilli and one with a lymph node pus positive for acid fast bacilli. Other patients had associated intracranial features like tuberculoma, meningeal enhancement, hydrocephalus, and vasculitis or the presence of disseminated TB as depicted in Supplementary Fig. S2 (online only).
PIM was made by clinical correlation with a history of preceding infection, a normal CSF, routine blood test, and autoimmune testing. A diagnosis of JE sequalae was made in a patient of JE with paraparesis whose CSF serology was positive for JE. Radiation myelitis (Supplementary Fig. S3, online only), spinal cord infarct, and ADEM (Supplementary Fig. S4, online only) diagnosis was made by radiological and clinical correlation. SLE was diagnosed in a young female with ANA positive having an end titer of 1:320 of speckled pattern.
MRI spine, brain, and optic nerve study was done in all patients and the important finding is depicted in Table 1. The most common lesion location was cervico-dorsal spine seen in 12 patients who included 6 NMO patients, 1 TB, 2 post-infectious, and 3 others. The most common site involved in NMO was cervico-dorsal spine (71%) and dorsolumbar spine in TB (50%). The lesion was predominantly central in both NMO (100%) and TB (80%) as compared with the other causes of LETM. More than 50% of the transverse area of the cord was noted in both NMO (71%) and TB (50%), but < 50% was more common in the post-infectious and others causes of LETM. However, entire cross-sectional area of the cord involvement was seen in 3 patients of the post-infectious group (42%). Cord expansion and patchy heterogeneous enhancement was seen in almost all patients. Spinal cord granuloma was seen in 1 patient with TB.
|
Features |
NMO (N = 7) |
TB (N = 6) |
Post-infection (N = 6) |
Others (N = 6) |
|
|---|---|---|---|---|---|
|
Cervical |
1 |
0 |
2 |
2 |
|
|
Dorsal |
1 |
2 |
1 |
0 |
|
|
Lesion location |
Cervico-dorsal |
5 |
1 |
2 |
3 |
|
Dorsolumbar |
0 |
3 |
0 |
0 |
|
|
Holocord |
0 |
0 |
1 |
1 |
|
|
Central |
7 |
5 |
1 |
3 |
|
|
Lesion distribution |
Peripheral |
0 |
1 |
2 |
2 |
|
Central and peripheral |
0 |
0 |
3 |
1 |
|
|
Well-defined homogeneous |
0 |
0 |
0 |
0 |
|
|
Contrast enhancement |
Patchy heterogeneous |
7 |
5 |
6 |
4 |
|
Ring enhancement |
0 |
1 |
0 |
1 |
|
|
> 50% |
5 |
3 |
1 |
1 |
|
|
Lesion area involved |
< 50% |
2 |
3 |
2 |
5 |
|
100% |
0 |
0 |
3 |
0 |
|
|
Syrinx |
0 |
1 |
1 |
0 |
|
|
Arachnoiditis |
0 |
5 |
0 |
0 |
|
|
Others |
Cord expansion |
5 |
5 |
5 |
5 |
|
Brainstem involvement |
1 |
0 |
1 |
0 |
|
|
Optic nerve involvement |
Unilateral |
0 |
1 |
0 |
0 |
|
Bilateral |
5 |
0 |
0 |
0 |
|
|
MS-like features |
2 |
0 |
0 |
0 |
|
|
ADEM features |
0 |
0 |
0 |
2 |
|
|
Brain involvement |
Vasculitis |
0 |
1 |
0 |
0 |
|
Granuloma |
1 |
1 |
0 |
0 |
|
|
Hydrocephalus |
0 |
1 |
0 |
0 |
|
|
Nonspecific |
0 |
0 |
0 |
0 |
Abbreviations: ADEM, acute disseminated encephalomyelitis; LETM, longitudinal extensive myelitis; MS, multiple sclerosis; NMO, neuromyelitis optica; TB, tuberculosis.
Associated brainstem involvement was seen in 2 patients, one NMO and the other in the post-infectious group. Syrinx was seen in two patients; one was TB and the other was JE sequalae. Anterior horn cell involvement was seen in the post-JE sequalae patient (Supplementary Fig. S5, online only). Optic nerve involvement was seen in 6 patients, 5 patients belong to the NMO group and were bilateral in nature, and 1 was unilateral in the TB group.
Brain MRI was abnormal in 3 patients of NMO, 2 had MS-like demyelinating features (Supplementary Fig. S1, online only) and the other one had nonspecific features. ADEM group had an abnormal diffuse white matter brain lesion in both the patients. The TB group has abnormal MRI brain findings like arachnoiditis (80%), vasculitis (16%), hydrocephalus (14%), and conglomerated ring enhancing lesion (14%) as shown in Supplementary Fig. S2 (online only).
Discussion
Longitudinal myelitis is a presentation of ATM in all age group.10 The exact incidences and prevalence of longitudinal myelitis in India is not known. In South India, the prevalence of MS was noted as 8.3/100,000, and the prevalence of NMOSD which is an important cause of LETM was 2.6/100,000 among all demyelinating disorders.11 12 In one study conducted by Pandey et al13 in North India, they studied 80 patients out of which LETM was one of the most common presentation. Another study conducted in North East India which included 151 patients of noncompressive myelopathy with 35 years as their median age and a sex ratio of 1.4:1 showing a male predominance.14 In the present series, 28 years was our median age with a sex ratio of 1:2 showing a female predominance. Similar age and gender profile were observed in other previous studies.15 16 17
The clinical symptom further depends on the type of myelitis (partial or complete) and the area of the cord involved whether cervical, cervicothoracic, dorsal, dorsolumbar, or the entire length. Pandey et al13 noted that majority (n = 66, 82.5%) of his patients had longitudinally extensive transverse myelitis. Eleven patients had cervical myelitis, 32 patients had cervicothoracic myelitis, 24 patients had thoracic myelitis, and 13 patients had whole cord myelitis. Centro-medullary involvement was more frequent in the idiopathic transverse myelitis group. Another study concluded acute myelitis in 66.3% and chronic myelitis in 33.6% among their group study of 151 patients of noncompressive myelopathy.14 In the present series, paraparesis (84%) was the most common clinical manifestation, with acute presentation in 40%, subacute in 31%, and chronic in 28% of patients. The most common location among the diagnosed case was cervico-dorsal cord (12 patients), cervical (5 patients), dorsal (4 patients), and dorsolumbar in 3, with the entire cord involvement in 2 patients.
The various etiology of noncompressive myelopathy depends on the clinical presentation. Some causes are associated with relapse and remitting course whereas some has a monophasic phase. NMO has gained its own importance among other longitudinal myelitis cases. It can have a recurrent presentation or as a single monophasic form. Presence of optic neuritis and a longitudinal myelitis are absolute criteria but presence of two additional criteria like involvement of three or more than three vertebral segments in MRI spine and brain findings not suggestive of MS and an AQPo4 seropositivity.18
Kayal et al noted LETM in 63.8% out of their noncompressive myelopathy group. The most common etiology noted was NMO seen in 23 patients. Other were infectious causes, metabolic, PIM, radiation myelitis, AV dural fistula, ADEM, paraneoplastic, autoimmune, and unknown in 24 patients.14 Pandey et al noted in their series of ATM that idiopathic was the most common etiology seen in 61.25%. The next major group of disorders was NMOSD and others were MS, infectious myelitis, and post-infectious case.10 In another study done retrospectively on LETM patients in North West India, 21 patients (32.81%) out of 64 were clinically diagnosed as NMO but only 13 patients were seropositive. Other etiologies seen were MS, ADEM, post-infectious, subacute combined degeneration of spine, AV malformation, SLE, and TB of spine. Diagnosis could not be made in 9 patients.19 In another retrospective, multicentric study of 27 patients of LETM, the various diagnosis made were NMOSD which was the most common seen in 37% followed by idiopathic seen in 22.2%. Others were SLE, AV fistula, ADEM, and MS.20 Only 1 to 2% of patients with SLE develop lupus myelitis which can be complete or partial.21
In the present series of longitudinal myelitis, NMO was the most common etiology noted in 7 patients (21.8%) followed by tuberculous infection of the spine seen in 6 patients (18.7%) and PIM in 6 patients (18.7%). The other etiologies noted was ADEM (6.24%), radiation myelitis (6.24%), spinal cord infarct (3.12%), SLE seen in 1 patient (3.12%), and JE sequalae seen in one. Diagnosis could not be labeled in 6 patients. This finding of TB being the second most common cause of LETM differs from other study. This could be because of the higher prevalence of extrapulmonary TB in this area of the country as compared with the rest.22 23 However, a significant seasonal variation in TB is observed in North India and North Eastern India.24 T2 hyperintensities of the cervical and dorsal cord are relatively more common as compared with lower spinal regions in NMO.25 In the present study, the most common location was cervico-dorsal (71%). NMO lesions are located in the central area involving > 50% of the transverse area of the cord.26 Although these lesions are known for its central gray matter location,27 few studies have noted it can bear both central and peripheral location.28 However, > 50% axial cross-section cord involvement was noted to have a high sensitivity (93.8%) but its low specificity (30.4%) has left an area of delima.28
In the present study, all NMO showed lesions with central location but the infectious group (TB) also had centrally distributed lesion in 80% cases. NMO and TB have a transversally extensive lesion (> 50%) in most of their cases but the entire cross-section of the cord transversally was involved in 2 patients belonging to the post-infectious group. Another study involving 15 patients of NMO noted that AQPo4 seropositivity was highly associated with certain clinical and radiological features like female sex, presence of cord expansion, bright spotty lesion, and a cervicomedullary area involvement.29 Certain MRI features of the spine described as “bright spotty lesions” are noted to be highly specific and a distinctive feature of NMO helping us to differentiate it from other LETM etiologies.28 Abnormal brain MRI in NMO patients is noted in up to 50 to 85% of cases. These lesions are usually periventricular in location (3rd and 4th ventricle), nonspecific white matter changes, and also midbrain and cerebellum lesions can be seen.30 31 We also noted abnormal brain MRI in three patients, one had nonspecific findings and two had MS-like features.
Tubercular myelitis is a rare presentation usually involving the dorsal cord.32 Gupta et al42 noted that most of the lesions in tuberculous myelopathy were hypointense to isointense in T1 and hyperintense on T2 lacking enhancement after contrast administration. Another retrospective study analyzed the neuroimaging of 10 patients of tubercular myelitis, involvement of cervical/thoracic segments was most commonly seen (90%) with enhancement after contrast administration seen only in 6 and epidural enhancement in 2 patients only.33 In the present study, dorsolumbar region (50%) was mostly affected by TB. All the patients showed enhancement after contrast, patchy enhancement in 5 patients and ring enhancing lesion in 1 patient. Arachnoiditis was seen in 5 patients and cord swelling in 5 patients, 1 patient had a syrinx. The lesion was mostly central in location (80%) with > 50% cross-section area of cord involved in half of the cases. Two-thirds involvement of the transverse area of the cord has been noted in other studies also.34 Brain MRI is useful to rule out associated brain TB or confirmation of isolated tubercular myelitis involvement.35 In the present series, abnormal brain MRI findings like vasculitis, hydrocephalus, meningitis, and ring-enhancing granuloma added to the diagnosis.
Neuroimaging noted that a simultaneous bilateral posterior involvement of the optic nerve and chiasm showing nonspecific sheet thickening and hyperintensity with contrast enhancement is more classical of NMOSD.36 Bilateral retrobulbar intraorbital segment of the optic nerve was involved in 5 patients of NMO in the present study. However, the same was noted in one patient of TB, which was unilateral enhancement along the anterior and posterior aspect of the optic nerve. Different studies reported vision impairment incidence of 27 to 72% in central nervous system TB as a result of chiasm involvement due to arachnoiditis or compression by a tuberculoma. Optic nerve involvement by compression by a tuberculoma or a tuberculoma itself.37 A study from North East India on tubercular involvement of the brain, noted that the optic nerve was the most common cranial nerve involved with visual loss noted in 6 patients.38
Several reports observed that LETM was typically seen in children with ADEM.39 The classical brain MRI picture of bilateral diffuse subcortical and deep white matter symmetric involvement is helpful in making the diagnosis. We noted two patients of ADEM whose MRI brain were classical.
PIM radiological findings were not classical and nonspecific. In the present study, we noted whole length of cord involvement in 1 patient (14%) and whole cross-sectional area involvement in the post-infectious group in 3 patients (42%). The lesion was located both centrally and peripherally with no specific involvement. Post-JE vaccination myelitis is a known fact but JE infection myelitis or as a sequalae is rarely reported.40 41 We also report a JE infection with myelitis features, where multiple foci of T2/STIR hyperintensities are seen within the cord with hyperintensities also noted within anterior horn cells along with syrinx in the cervical and dorsal cord.
Conclusion
Longitudinal myelitis has a varied list of etiological diagnosis. Infection and parainfectious causes have to be kept in mind to differentiate it from NMO as both have a different course of treatment and prognosis. Central cord involvement and > 50% cross-section area involvement can be seen both in NMO and the infectious group, but cervico-dorsal location is more common in NMO and dorsal cord in TB. Abnormal brain MRI is less common but with specific findings in NMO but more common in TB and ADEM.
Conflict of Interest
None declared.
Funding None.
References
- Diagnosis and differential diagnosis of acute transverse myelopathy. The role of neuroradiological investigations and review of the literature. Neurol Sci. 2001;22(02):S69-S73.
- [Google Scholar]
- Aquaporin 4 IgG serostatus and outcome in recurrent longitudinally extensive transverse myelitis. JAMA Neurol. 2014;71(1):48-54.
- [Google Scholar]
- Conventional and advanced imaging in neuromyelitis optica. AJNR Am J Neuroradiol. 2014;35(8):1458-1466.
- [Google Scholar]
- Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59(4):499-505.
- [Google Scholar]
- International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261-1267.
- [Google Scholar]
- Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
- [Google Scholar]
- International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189.
- [Google Scholar]
- Pediatric acute transverse myelitis overview and differential diagnosis. J Child Neurol. 2012;27(11):1426-1436.
- [Google Scholar]
- Multiple sclerosis in India: an overview. Ann Indian Acad Neurol. 2015;18(01):S2-S5.
- [Google Scholar]
- Prevalence and patterns of demyelinating central nervous system disorders in urban Mangalore, South India. Mult Scler. 2014;20(12):1651-1653.
- [Google Scholar]
- Etiologic spectrum and prognosis in noncompressive acute transverse myelopathies: an experience of 80 patients at a tertiary care facility. Neurol India. 2018;66(1):65-70.
- [Google Scholar]
- Etiological profile of noncompressive myelopathies in a tertiary care hospital of Northeast India. Ann Indian Acad Neurol. 2017;20(1):41-50.
- [Google Scholar]
- Profile of non-compressive myelopathy in eastern India: a 2-year study. Acta Neurol Scand. 1999;99(2):100-105.
- [Google Scholar]
- Non-compressive myelopathy: clinical and radiological study. Neurol India. 1999;47(4):294-299.
- [Google Scholar]
- Patterns of non-traumatic myelopathies in Yaounde (Cameroon): a hospital based study. J Neurol Neurosurg Psychiatry. 2010;81(7):768-770.
- [Google Scholar]
- Longitudinally extensive transverse myelitis: A retrospective analysis of sixty-four patients at tertiary care center of North-West India. Clin Neurol Neurosurg. 2016;148:5-12.
- [Google Scholar]
- Diagnósticosdiferenciales y pronóstico de las mielitislongitudinalesextensasen Buenos Aires, Argentina. Neurologia. 2017;32:99-105.
- [Google Scholar]
- Transverse myelitis as a presenting feature of late onset systemic lupus erythematosus. Ann Saudi Med. 2009;29(2):156-157.
- [Google Scholar]
- TB India 2018: Annual Status Report. New Delhi: Central TB Division; 2018. . Accessed May 27, 2018 at
- [Publisher] [Google Scholar]
- Tuberculosis in North-East India: patient profile and treatment outcome of patient attending RNTCP. Int J Community Med Public Health. 2019;6(7):2856-2860.
- [Google Scholar]
- Analyzing seasonality of tuberculosis across Indian states and union territories. J Epidemiol Glob Health. 2015;5(4):337-346.
- [Google Scholar]
- Modifications of longitudinally extensive transverse myelitis and brainstem lesions in the course of neuromyelitis optica (NMO): a population-based, descriptive study. BMC Neurol. 2013;13:33.
- [Google Scholar]
- “Bright spotty lesions” on spinal magnetic resonance imaging differentiate neuromyelitis optica from multiple sclerosis. Mult Scler. 2014;20(3):331-337.
- [Google Scholar]
- Preferential spinal central gray matter involvement in neuromyelitis optica. An MRI study. J Neurol. 2008;255(2):163-170.
- [Google Scholar]
- Differentiating neuromyelitis optica from other causes of longitudinally extensive transverse myelitis on spinal magnetic resonance imaging. Mult Scler. 2016;22(3):302-311.
- [Google Scholar]
- MRI features of aquaporin-4 antibody-positive longitudinally extensive transverse myelitis: insights into the diagnosis of neuromyelitis optica spectrum disorders. AJNR Am J Neuroradiol. 2018;39(4):782-787.
- [Google Scholar]
- Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14.
- [Google Scholar]
- An uncommon cause of longitudinally extensive transverse myelitis. Maedica (Bucur). 2016;11(3):245-249.
- [Google Scholar]
- Neuroimaging of tuberculous myelitis: analysis of ten cases and review of literature. J Neuroimaging. 2006;16(3):197-205.
- [Google Scholar]
- Tuberculous transverse myelitis case report and review of the literature. Clin Pulm Med. 2005;12(1):46-52.
- [Google Scholar]
- Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol. 2012;32(3):216-220.
- [Google Scholar]
- Invasion of the brain and chronic central nervous system infection after systemic Mycobacterium avium complex infection in mice. Infect Immun. 2000;68(5):2979-2984.
- [Google Scholar]
- Clinical and radiological spectrum of intracranial tuberculosis: a hospital based study in Northeast India. Indian J Tuberc. 2017;64(2):109-118.
- [Google Scholar]
- Idiopathic acute transverse myelitis in children: an analysis and discussion of MRI findings. Mult Scler. 2011;17(1):74-80.
- [Google Scholar]
- Acute transverse myelitis following Japanese encephalitis viral infection: an uncommon complication of a common disease. BMJ Case Rep. 2012;2012:bcr2012007094.
- [Google Scholar]
- Acute transverse myelitis after Japanese B encephalitis vaccination in a 4-year-old girl. Brain Dev. 2002;24(3):187-189.
- [Google Scholar]






