Translate this page into:
Estimation of cerebrospinal fluid cortisol level in tuberculous meningitis
Address for correspondence: Dr. Rohan R. Mahale, Department of Neurology, M. S. Ramaiah Medical College and Hospital, Bengaluru - 560 054, Karnataka, India. E-mail: rohanmahale83@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Central nervous system (CNS) involvement in tuberculosis is around 5–10%. Of the various manifestations of CNS tuberculosis, meningitis is the most common (70–80%). Delay in diagnosis and treatment results in significant morbidity and mortality.
Objective:
To study the cerebrospinal fluid (CSF) cortisol levels in tubercular meningitis and compare the levels with controls.
Methods:
Cross-sectional, prospective, observational, hospital-based study done in 20 patients of tubercular meningitis, 20 patients of aseptic meningitis (AM) and 25 control subjects without any preexisting neurological disorders who have undergone lumbar puncture for spinal anesthesia.
Results:
Cortisol was detected in all 40 CSF samples of patients (100%). Mean CSF cortisol level was 8.82, 3.47 and 1.05 in tubercular meningitis, AM and controls, respectively. Mean CSF cortisol level in tubercular meningitis was significantly higher as compared to AM and controls (P < 0.0001).
Conclusion:
Cortisol level estimation in CSF is one of the rapid, relatively inexpensive diagnostic markers in early identification of tubercular meningitis along with CSF findings of elevated proteins, hypoglycorrhachia and lymphocytic pleocytosis. This aids in earlier institution of appropriate treatment and thereby decreasing morbidity and mortality. This is the first study on the estimation of CSF cortisol level in tuberculous meningitis.
Keywords
Cerebrospinal fluid
cortisol
marker
meningitis
tuberculous
Introduction
Tuberculosis remains a major public health problem in developing countries,[1] mostly seen in age group of 15–59 years. However, increasing incidence of human immune deficiency virus, there is a potential for re-emergence of tuberculosis as a significant public health problem in the developed countries as well.[2] Of the various manifestations of central nervous system tuberculosis, meningitis is the most common (70–80%) followed by tuberculoma.[3] Tuberculous meningitis (TBM) is associated with significant neurological sequelae, high morbidity and mortality.[4] Clinical suspicion supported by careful cerebrospinal fluid (CSF) analysis is the only method even today for the diagnosis of TBM. CSF analysis plays a pivotal role in the diagnosis of TBM. Contemplated confirmatory diagnostic tests based on the detection of the mycobacterium for TBM includes CSF smear for acid fast bacilli (AFB) and CSF culture for AFB. Demonstration of AFB in CSF is the single most important procedure for a definitive diagnosis. CSF smear for AFB is positive in 5–37% cases and the CSF culture for AFB is positive in 40% cases.[56] It is estimated that for demonstration of AFB on smear, bacterial load of 10,000 AFB/ml is required and for culture to be positive; there shall be 100 AFB/ml of CSF. It is tedious, and time-consuming to grow tubercle bacilli on culture requiring 3–8 weeks. Polymerase chain reaction (PCR) test is a highly sensitive and specific test, but is very costly and not widely available.[78] Hence, newer rapid, relatively inexpensive diagnostic markers in CSF are required for the diagnosis of TBM. The objective of this study was to study the CSF cortisol levels in TBM in comparison with aseptic meningitis and controls.
Methods
Patients and setting
A total of 25 patients clinically suspected of tubercular meningitis and 20 patients clinically suspected of aseptic meningitis, who were admitted in M.S Ramaiah hospitals, Bangalore, India were recruited for the study. The study period was from July 2009 to July 2011 and was approved by the institute ethics committee. All subjects or their caregivers gave written informed consent after full explanation and detailed description of study method. The inclusion criteria included age 18 years or greater, patients presenting with fever, headache and meningeal irritation and lumbar puncture performed upon admission to the hospital. The Medical Research Council (MRC) staging of TBM:[9] Stage 1: Prodromal phase with no definite neurological symptoms or signs; Stage 2: Signs of meningeal irritation with slight or no clouding of consciousness and minor (cranial) nerve palsies or neurological deficits; Stage 3: Severe clouding of consciousness, stupor, coma, convulsions, gross paresis or involuntary movements were carried out in all included patients. Patients with a predisposition for bacterial meningitis such as bacterial sinusitis, otitis media, CSF rhinorrhea and patients with history of head injury and nasal or ear bleeding, partially treated cases (which included antibiotics and steroids) were excluded from the study. Five patients met the exclusion criteria and were excluded from the study. 25 patients (15 males and ten females) without any preexisting neurological disorders who underwent spinal anesthesia were included as controls. All patients and controls were examined by one of the authors (A.M.). The study was prospective, cross-sectional, observational and hospital-based.
Investigations
All the patients underwent thorough clinical examination. The following information was documented in the predesigned proforma: Characteristics of fever, headache, level of consciousness, details about convulsions, focal neurological deficits, history of surgeries and co-morbidities such as diabetes, pulmonary or extra-pulmonary tuberculosis. Following investigations were carried out in all the patients that include complete blood count, renal, hepatic function tests, serum electrolytes, random blood sugar: At the time of lumbar puncture, chest X-ray, abdominal ultrasonography, blood culture, CSF study: Cytochemical analysis-cell count, cell type, protein, sugar, cortisol estimation by chemiluminescent method, gram stain and ZN stain, culture for mycobacteria and bacteria, TB PCR, Herpes Simplex virus PCR, India ink preparation, cryptococcal antigen and malignant cell cytology. Neuroimaging in the form of the computed tomography head or magnetic resonance imaging brain was done in all patients to rule out structural brain lesion before lumbar puncture. CSF examination was done as soon as a patient got admitted before the first dose of antibiotics or steroids were given.
Analysis of cerebrospinal fluid-cortisol level
A fresh sample of CSF obtained by the lumbar puncture was collected in heparinized vial and analyzed for the cortisol estimation by direct chemiluminescence assay using ADVIA Centaur CP system. The ADVIA Centaur CP cortisol assay is a competitive immunoassay using direct chemiluminescent technology. Cortisol in the patient sample competes with acridinium ester labeled the cortisol in the Lite Reagent for binding to polyclonal rabbit anticortisol antibody in the solid phase. The polyclonal rabbit anticortisol antibody is bound to monoclonal mouse antirabbit antibody, which is covalently coupled to paramagnetic particles in the solid phase.
The following CSF cytochemical parameters for TBM were used: Protein: 1–5 g/L, cell counts: 10–500 cells/µL; lymphocytic predominance; glucose: 20–40 mg/dl.[10]
Statistical analysis
The data were analyzed using SPSS 17 (IBM inc.). The continuous variables were expressed as mean ± standard deviation and categorical variables as frequency and percentage. The normality of the distribution was assessed by the skewness of the values. For the analysis of continuous variables, nonparametric testing (Mann–Whitney test and Wilcoxon's test) was employed. P < 0.05 was taken as statistically significant.
Results
Demographic and clinical data
A total of 20 patients clinically suspected of TBM were recruited during the study period. Mean age of patients with meningitis was 45.6 ± 17.4 years (range: 21–68). There was slightly higher proportion of males (n = 12) as compared to females (n = 08) with M: F ratio of 1.5:1. The most common clinical symptom was fever (20/20), headache (16/20), altered level of consciousness (16/20) and convulsions (8/20). The most common clinical signs were neck stiffness (20/20), drowsiness (17/20), irritability (15/20), stupor (5/24), cranial nerve deficits (15/30) and hemiparesis (8/20). The duration of symptoms was < 1 week in 3 (15%), 1–3 weeks in 12 (60%), >3 weeks in 5 (25%) patients.
Clinical course of patients with tubercular meningitis
A total of 20 patients presented with tubercular meningitis. CSF TB-PCR was positive in 6 patients. CSF culture was positive for mycobacterial growth in four patients. Six patients had pulmonary tuberculosis, and eight patients had tubercular lymphadenitis. Three patients were in stage 1 (3/20), twelve patients in stage 2 (12/20) and five patients in stage 3 (5/20) according to MRC staging of TBM. Favorable outcomes of tubercular meningitis were observed in 15 patients (75%) with three patients having focal neurological deficits due to tubercular arteritis (15%), two patients had obstructive hydrocephalous requiring CSF diversion procedures (10%).
Clinical course of viral meningitis
A total of 20 patients had CSF features suggestive of viral meningitis. CSF HSV PCR was positive in eight patients; varicella-zoster virus PCR was positive in five patients and unknown etiology in seven patients. Favorable outcomes of viral meningitis were observed in all 16 patients (80%).
Cytology and chemistry of cerebrospinal fluid
Serum and CSF cytological and clinical chemistry parameters in the tubercular meningitis and aseptic meningitis are summarized in Table 1.
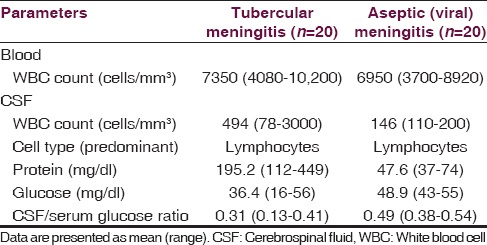
Cortisol level in cerebrospinal fluid
Cerebrospinal fluid cortisol levels in tubercular meningitis, aseptic meningitis and controls are summarized in Table 2. The mean CSF cortisol level in tubercular meningitis group was significantly higher as compared to aseptic meningitis and control groups (P < 0.0001).

Discussion
The present study was aimed at determining the CSF cortisol levels in TBM. Twenty patients with TBM and aseptic meningitis were studied, and CSF cortisol levels were assessed in them before institution of treatment. CSF cortisol levels in both groups were compared with the control group. CSF cortisol levels were significantly elevated in patients with TBM as compared with concentrations in aseptic meningitis group and healthy control individuals. This is the first study determining the level of cortisol in CSF in patients with TBM.
Mycobacterial infection induces the production of proinflammatory cytokines that are important for bacilli killing, but they can also produce immunopathology.[11] At the early stage of mycobacterial infection of brain, proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL-1 and IL-6) are released by migrated activated macrophages, microglial cells, astrocytes, and endothelial cells. These cytokines are responsible for the damage to the blood brain barrier (BBB).[12] High concentrations of interferon-γ, and TNF-α have been detected in the CSF of TBM patients in correlation with the severity of the disease, and although these levels decreased after treatment, high levels were maintained at the end of 6 months of treatment.[131415]
Antinflammatory cytokines are also highly produced wherein; they protect tissue damage by excessive inflammation and induce nervous tissue regeneration.[11] Murine model of TBM-induced by the intratracheal infection showed high expression of antiinflammatory cytokines in the brain, such as IL-10, IL-4, and transforming growth factor-β, which apparently protect tissue destruction from excessive inflammation.[1617] Many studies have indicated an important role for cortisol as an anti-inflammatory response.[18]
Cortisol is a small lipid-soluble molecule produced in adrenal glands after stress stimulation such as infection, trauma or major surgery. It circulates in the blood mainly in the protein-bound forms, the majority being attached to corticosteroid binding globulin. The half-life of cortisol in the circulation is about 80 min, with approximately 1% excreted unchanged in the urine. Increase in cortisol secretion takes place very quickly, within minutes in acute stress conditions, and can stay at high levels for long periods, sometimes days, months, and even years.[19] It easily crosses the BBB. Schwarz and Pohl demonstrated that cortisol transport through BBB is not increased by its leakage and is rather dependent on serum concentrations.[20] It has been suggested that intrathecal concentrations of steroid hormones are maintained by their active transport out of the brain.[21] Disturbance of this mechanism by inflammation, with reduced ability of brain cells to metabolize sterol molecules, may lead to the persistent increase in CSF cortisol.[22] During critical illness, cortisol-binding globulin and albumin blood levels decrease by about 50%, leading to an increase in biological active free cortisol.
Holub et al., have shown elevated CSF cortisol levels in patients with bacterial meningitis, and it correlated with disease severity.[22] Similar elevation in CSF cortisol levels were seen in patients with aseptic meningoencephalitis (AM) suggesting an important role of cortisol in AM.[23] There are no studies on CSF cortisol levels in patients with TBM.
The strength of the study is the estimation of CSF cortisol level in patients with TBM immediately before starting empirical antibiotics or steroids. However, this study is not devoid of limitations. Smaller cohort of patients in TBM group in this study is one of the limitations. Correlation of CSF cortisol with disease severity and long-term outcome scores were not carried out. For rapid etiological diagnosis in meningitis, various CSF parameters along with a new parameter in the form of CSF cortisol assay must be combined. The results of the present study should be a basis for larger, prospective clinical study regarding utility of CSF cortisol as a diagnostic biomarker in TBM.
Conclusion
Cerebrospinal fluid cortisol levels were significantly higher in TBM group as compared to aseptic meningitis and control group. CSF cortisol levels can be used as a diagnostic marker in TBM pending its confirmation in larger cohort of TBM patients. This is the first study determining the level of cortisol in CSF in patients with TBM.
Acknowledgments
We thank the administration of M.S. Ramaiah medical college and hospital, Bangalore, India for giving permission to conduct the study.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Neurological and related syndromes in CNS tuberculosis. Clinical features and pathogenesis. J Neurol Sci. 1971;14:341-57.
- [Google Scholar]
- Clinicopathologic study of tuberculous meningitis in adults. Am Rev Tuberc. 1956;74:830-4.
- [Google Scholar]
- Neurological and systemic complications of tuberculous meningitis and its treatment at Auckland City Hospital, New Zealand. J Clin Neurosci. 2010;17:1114-8.
- [Google Scholar]
- Central nervous system tuberculosis with the acquired immunodeficiency syndrome and its related complex. Ann Intern Med. 1986;105:210-3.
- [Google Scholar]
- Diagnostic criteria for tuberculous meningitis and their validation. Tuber Lung Dis. 1994;75:149-52.
- [Google Scholar]
- Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax. 1992;47:270-5.
- [Google Scholar]
- Evaluation of Amplicor PCR for direct detection of Mycobacterium tuberculosis from sputum specimens. J Clin Microbiol. 1995;33:2582-6.
- [Google Scholar]
- Harrison's Principles of Internal Medicine. (18th ed). United States of America: The McGraw-Hill Companies, Inc; 2011.
- [Google Scholar]
- The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143-55.
- [Google Scholar]
- Cerebrospinal fluid cytokines in patients with tuberculous meningitis. Clin Immunol Immunopathol. 1997;84:171-6.
- [Google Scholar]
- Proinflammatory cytokine levels in the serum and cerebrospinal fluid of tuberculous meningitis patients. Neurosci Lett. 2008;2(436):48-51.
- [Google Scholar]
- A study of cytokines in tuberculous meningitis: Clinical and MRI correlation. Neurosci Lett. 2010;483:6-10.
- [Google Scholar]
- Specific bacterial genotype of Mycobacterium tuberculosis cause extensive dissemination and brain infection in an experimental model. Tuberculosis (Edinb). 2010;90:268-77.
- [Google Scholar]
- The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol. 2007;184:100-12.
- [Google Scholar]
- Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290-303.
- [Google Scholar]
- Cortisol is secreted episodically by normal man. J Clin Endocrinol Metab. 1970;30:411-22.
- [Google Scholar]
- Steroid hormones and steroid hormone binding globulins in cerebrospinal fluid studied in individuals with intact and with disturbed blood-cerebrospinal fluid barrier. Neuroendocrinology. 1992;55:174-82.
- [Google Scholar]
- Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol. 2002;14:753-9.
- [Google Scholar]
- Cortisol levels in cerebrospinal fluid correlate with severity and bacterial origin of meningitis. Crit Care. 2007;11:R41.
- [Google Scholar]
- Interferon-gamma and cortisol levels in cerebrospinal fluid and its relationship to the etiology of aseptic meningoencephalitis. Prague Med Rep. 2006;107:343-53.
- [Google Scholar]






