Translate this page into:
Epidemiological, clinical and prognostic profile of childhood acute bacterial meningitis in a resource poor setting
Address for correspondence: Dr. Bankole Peter Kuti, Department of Paediatrics and Child Health, Obafemi Awolowo University, Ile-Ife, Nigeria. E-mail: kutitherapy@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Childhood bacterial meningitis is a neurologic emergency that continues to kill and maims children particularly in developing countries with poor immunization coverage.
Objective:
This study set out to assess the hospital incidence, pattern of presentation, etiologic agents, outcome and determinants of mortality among the children admitted with bacterial meningitis at the Wesley Guild Hospital (WGH), Ilesa.
Patients and Methods:
We carried out a retrospective review of admitted cases of bacterial meningitis in children aged one month to 15 years at the WGH, Ilesa over a three year period by looking at the hospital records. Factors in the history and examinations were compared among survivors and those that died to determine factors significantly associated with mortality in these children.
Results:
Eighty-one (5.5%) of the 1470 childhood admissions during the study period had bacterial meningitis. Male preponderance was observed and two-thirds of the children were infants. More cases were admitted during the wet rainy season than during the dry harmattan season. Haemophilus influenzae type B and Streptococcus pneumoniae were the leading etiologic agents and ciprofloxacin and ceftriaxone adequately cover for these organisms. Twenty-two (27.2%) of the 81 children died, while 34 (42.0%) survived with neurologic deficits. Children with multiple seizures, coma, neck retraction, hyponatremia, hypoglycorrhachia, turbid CSF as well as Gram positive meningitis at presentation were found to more likely to die (P < 0.05). None of these factors however independently predict mortality.
Conclusion:
Childhood bacterial meningitis often results in death and neurologic deficit among infants and young children admitted at the WGH, Ilesa. Children diagnosed with meningitis who in addition had multiple seizures, neck retraction and coma at presentation are at increased risk of dying.
Keywords
Children
mortality
neurologic deficit
pyogenic meningitis
Introduction
Bacterial meningitis is a significant cause of childhood morbidity and mortality particularly in developing countries of sub-Saharan Africa.[1] The clinical manifestations of childhood meningitis are protean.[2] Fever, convulsion, headache, neck stiffness, vomiting, poor suck/feeding, and loss of consciousness though common in childhood meningitis, they are by no means pathognomonic of the infection.[23] In resource-poor regions, these symptoms are often initially ascribed to other childhood infections or thought to be due to supernatural causes resulting in late presentation and often unfavorable outcome.[4]
Although, there has been a lot of published works on the manifestations and etiologies of childhood meningitis in Nigeria[5678] and other developing countries;[910] there is, however, paucity of studies on the determinants of meningitis-related mortality. Also, frequent review of the bacterial causes of childhood meningitis and their antibiotic sensitivity pattern is desirable to identify changes and/or trends if any. This study therefore aims to determine the pattern of presentation, causes, and determinants of mortality of children admitted with bacterial meningitis at the Wesley Guild Hospital (WGH), Ilesa.
Patients and Methods
Study design
This is a retrospective review of admitted cases at the pediatric wards of the WGH which is a tertiary annexe of the Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife. The hospital receives referrals from both private and public primary and secondary health facilities and serves the health needs of the communities of Osun, Ondo, and Ekiti States of the South-West Nigeria. It is one of the main referral centers providing specialized pediatric services for these communities. The children emergency ward of the hospital operates a 24-h service and admits about 600 children per annum. The hospital has functional biochemical, microbiological, and hematological laboratory services as well as well-equipped and staffed radiological services which also operate on a 24 h basis. Ilesa, the largest town in Ijesaland is situated on latitude 7°35’N and longitude 4°51’E and is about 200 km North-East of Lagos a major commercial nerve center of Nigeria.[11] Ilesa is home to about 620,000 people with about 25% of the population being children <5 years and up to 40% children <15 years.[11] The people in Ilesa called Ijesa are mainly traders, peasant farmers, artisans, and civil servants.[11]
Methods
The records of children aged 1-month to 15 years admitted and managed for bacterial meningitis over a 3-year study period (January 2011 to December, 2013) were retrieved. Bacterial meningitis was defined in this study as positive bacterial growth on culture of the cerebrospinal fluid (CSF) and/or Gram stain reaction with the presence of five or more pus cells in the CSF. Also, CSF polymorphonuclear pleocytosis with elevated CSF protein (in excess of 45 mg/dl) and low CSF glucose (hypoglycorrhachia) less than one-half of blood glucose was also considered as bacterial meningitis.[2] Children with tuberculous, aseptic or viral meningitis and those <1-month were excluded. For this study, hypoglycemia was defined as random blood glucose <2.2 mmol/L, acidosis as serum bicarbonate level <20.0 mmol/L and hyponatremia was defined as serum sodium <135 mmol/L and anemia as hematocrit <30%.
The study variables included the age, sex, immunization status of the subjects, and parental occupation as well as the highest level of education from which the socioeconomic class of the parents were obtained.[12] Presenting features including fever, poor sucking/feeding, convulsion, vomiting, and their duration were noted as well as history of previous hospital admission. Coma at presentation, the presence of neck stiffness, signs of meningeal irritation, and other findings on system examination were documented.
Laboratory results of note included CSF examination - the appearance, the protein, glucose, microscopic, culture, and sensitivities. Also, serum electrolytes particularly sodium and bicarbonate were noted. Random blood sugar and blood culture, and sensitivities results were also noted.
The patients were managed according to the standard protocol of the unit including the administration of intravenous ampicillin and chloramphenicol or ceftriaxone, fluid and calorie maintenance, anticonvulsants, and other supportive management were ensured. Outcome of hospitalization was categorized into discharged home well, discharged with neurologic sequelae and death.
Approval for the study was obtained from the Ethics and Research Committee of the OAUTHC, Ile-Ife.
Data analysis
Data were analyzed using SPSS for Windows software version 17.0 (SPSS Inc. Chicago 2008). Differences between the means or median values of continuous variables were determined using Student's t-test or Mann–Whitney U-test; while the differences between proportions of categorical variables were determined using Pearson's Chi-squared or Fisher's exact tests. The level of significance at a 95% confidence interval was set at P < 0.05. Associations between mortality and independent variables that gave significant results were further analyzed with binary logistic regression to determine the independent predictors of mortality among the children with bacterial meningitis.
Result
Over the 3 years under review, 92 (6.3%) of the 1470 total admissions outside the neonatal period had meningitis. Eleven children were excluded because 7 (0.5%) had suspected viral meningitis and 4 (0.3%) had tuberculous meningitis. Therefore 81 (5.5%) of the 1470 total admission during the study period had bacterial meningitis and form the basis of further data analysis. Fifty-seven (62.7%) of the 81 children with meningitis had bacterial growth from their CSF culture while the diagnosis of meningitis was made by Gram stain and suggestive CSF biochemistry (Hypoglycorrhachia and elevated CSF protein) in 24 (37.3%) of the children. No outbreak of meningitis epidemic was reported during the study period in the region.
Sociodemographic characteristics of the children with meningitis
The age of the children with meningitis ranged from 1-month to 144 months with a median (interquartile range [IQR]) of 8.0 months (5.0–25.5 months). About two-third of the children were infants and 68 (84.0%) of the children <5 years. There was male preponderance with male to female ratio 1.4:1.
About one-half of the children with meningitis were from middle social class with parents having secondary education and are petty traders/artisans. About a third of these children were from lower social class, while none of the parents of these children were gainfully employed professionals in upper social class one. In addition, 27 (33.3%) of the children managed for pyogenic meningitis live in overcrowded houses.
Monthly distribution of the cases
The distribution of the monthly admission of the cases of childhood bacterial meningitis revealed peak incidences in the months of April and July. There were more cases of bacterial meningitis in the rainy season of April to September (58.0%) than during the dry months of October to March (42.0%), though the difference is not statistically significant (P = 0.720) [Figure 1].
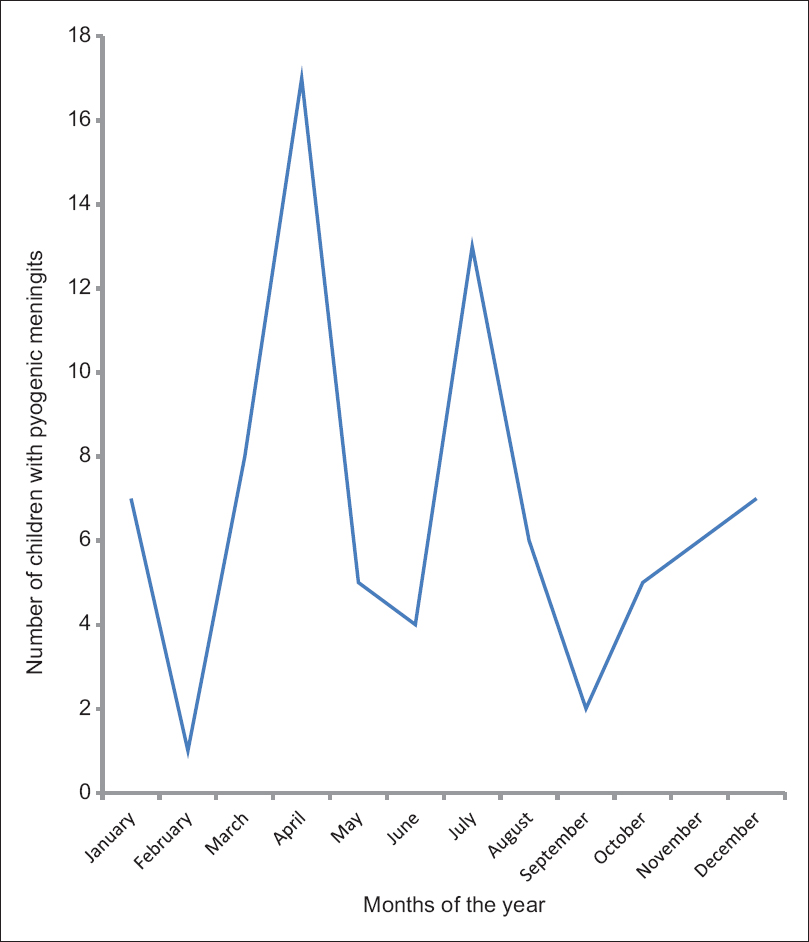
- Monthly distribution of the cases of pyogenic meningitis seen at the Wesley Guild Hospital from 2011 to 2013
Presenting clinical features
The weight of the children ranged from 2.9 to 33.0 kg with a median (IQR) of 7.3 (6.0–10.4) kg. Twenty-three (28.4%) of the children were underweight, 3.0 (3.7%) were marasmic, while the rest 55 (67.9%) had normal weight for age.
The mean temperature of the children was 37.9 (0.9°C) ranged from 35.6°C to 39.3°C. Majority (81.5%) of the children presented with fever, while 11 (13.6%) had normal temperature at admission, 4 (4.9%) had subnormal temperature.
Duration of symptoms before presentation ranged from 1 to 30 days with a median (IQR) of 4.0 (2.0–7.0) days. Majority (80.3%) of the children presented within 1-week of the illness.
[Table 1] shows the age distribution of the children with bacterial meningitis as related to the clinical features at presentation. Vomiting and neck stiffness were significantly found as presenting features among older children compared to those <5 years (P < 0.05). Conversely, irritability was significantly more common among under-fives. Seizures were observed in 61 (75.3%) of the children which was multiple in 43 (59.3%) of those with seizures. Twenty (24.7%) of the children had no seizure at presentation and throughout the duration of admission. Forty-two (51.9%) of the children with bacterial meningitis were unconscious at presentation. Though more proportion of children <5 years presented with coma compared to children older than 5 years, the difference was not significant (P = 0.654) [Table 1].
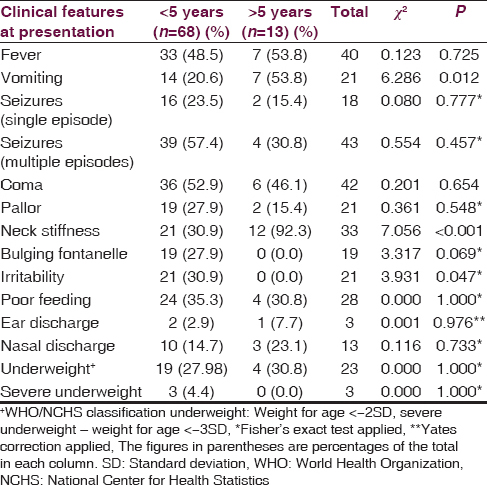
Laboratory findings at presentation
The laboratory findings at presentation among the children with meningitis as related to their age distribution are shown in Table 2. The mean (standard deviation [SD]) blood sugar of the children was 6.1 (2.3) mmol/L. This is ranged from 1.3 to 12.6 mmol/L. Eight (9.8%) of the children had hypoglycemia while 24 (29.6%) had hyperglycemia. The rest (60.6%) had normal blood sugar at presentation.
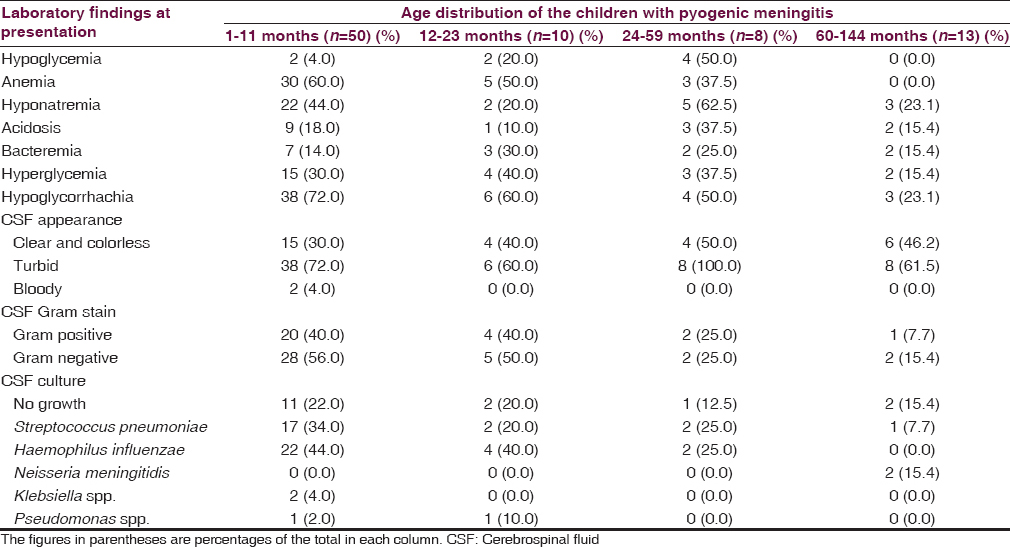
The serum sodium of the children ranged from 129 to 142 with a mean (SD) of 123.5 (23.3) mmol/L. Thirty-two (39.5%) of the children had hyponatremia (serum sodium <130 mmol/L), while 5 (6.1%) had hypernatremia (serum sodium > 150 mmol/L); the rest (54.4%) had normal serum sodium.
Serum bicarbonate ranged from 15 to 25 mmol/L with a mean (SD) of 20.4 (2.3) mmol/L. Fifteen children (18.5%) had metabolic acidosis.
Cerebrospinal fluid analysis
All the children had lumbar puncture for CSF analysis at admission. The CSF was turbid or cloudy in 70 (86.4%), clear and colorless in 9 (10.8%) and hemorrhagic in 2 (2.4%).
The CSF white cell count ranged from 2 to 2280 cells/mm3 with a median (IQR) count of 17 (8–25) cells/mm3.
Sixty-five (80.2%) of the 81 children with bacterial meningitis had reactive Gram staining including 37 (56.9%) Gram-negative and 28 (43.1%) Gram-positive. Eight children had reactive Gram stain even without any growth on culture.
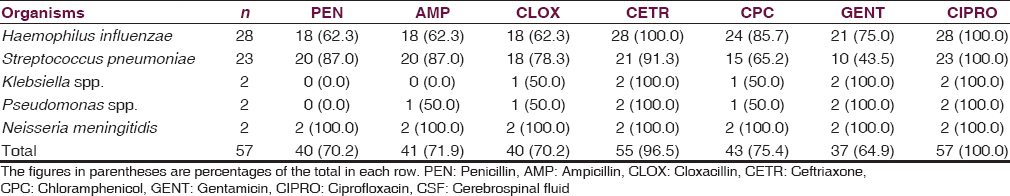
Fifty-seven (62.7%) of the 81 children had a positive bacterial yield on CSF culture. The predominant bacterial isolates were Haemophilus influenzae type B and Streptococcus pneumoniae. Table 2 highlights the distribution of the bacterial causes of meningitis in the children as related to age distribution. S. pneumoniae and H. influenzae type B predominates in infancy and children <5 years, while Neisseria meningitidis was isolated in school age children. The in vitro antibiotic sensitivity pattern to the isolated organisms is shown in Table 3.
The CSF protein of the children with pyogenic meningitis ranged from 8.0 to 846 with a median (IQR) of 170 (110–312) mg/dl. Seventy-nine (97.5%) of the children had elevated CSF protein (>45 mg/dl). The mean (SD) CSF glucose was 1.7 (4.18), median (IQR) of 0.65 (0.4–1.9). This is ranged from 0.1 to 30.0 mmol/L. Fifty-one (62.9%) had low CSF glucose (hypoglycorrhachia).
Blood culture
Only 35 of the 81 children had blood culture done at presentation before the commencement of antibiotics. 14 (40.0%) of these had bacteria growth on blood culture. The major organisms were H. influenzae type B 8 (12.3%), S. pneumoniae 6 (7.4%), Staphylococcus aureus 1 (1.2%), and Escherichia coli 1 (1.2%). Nine (11.1%) children had same organisms isolated from both blood and CSF, while an infant had S. aureus isolated from the blood and H. influenzae from the CSF.
Outcome of hospitalization
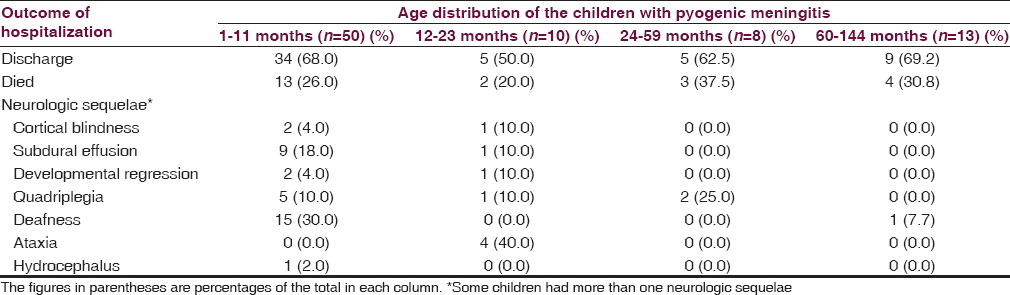
Twenty-two (27.2%) of the 81 children were died. Fifty-four (66.6%) of these children were discharged including 26 (32.1%) with neurologic deficit, 4 (4.9%) discharged against medical advice while 1 (1.2%) was referred to the neurosurgical unit. The age distribution of the children in relation to the outcome of hospitalization is highlighted in Table 4.
Neurologic deficit
The deficit observed among the 22 children included quadriplegia 8 (30.7%) of those with deficit, developmental regression 6 (23.1); ataxia 4 (15.4), deafness 4 (15.4); cortical blindness 3 (11.5%), and hydrocephalus 1 (3.8%). Five children had more than one type of deficits. Significantly more proportion of infants had neurologic sequelae at discharge compared to older age group (97.1 vs. 55.0; df = 1; χ2 = 12.109; P = 0.001) [Table 5].
Duration of hospital stay
The median (IQR) duration of hospital stay was 6.0 (4.0–11.0) days. This ranged from 1 to 28 days. There was a negative correlate between the age of the children and duration of hospital stay but this relationship was not statistically significant (Spearman rho = −0.172; P = 0.144).
Sociodemographic characteristics of the patients in relation to mortality
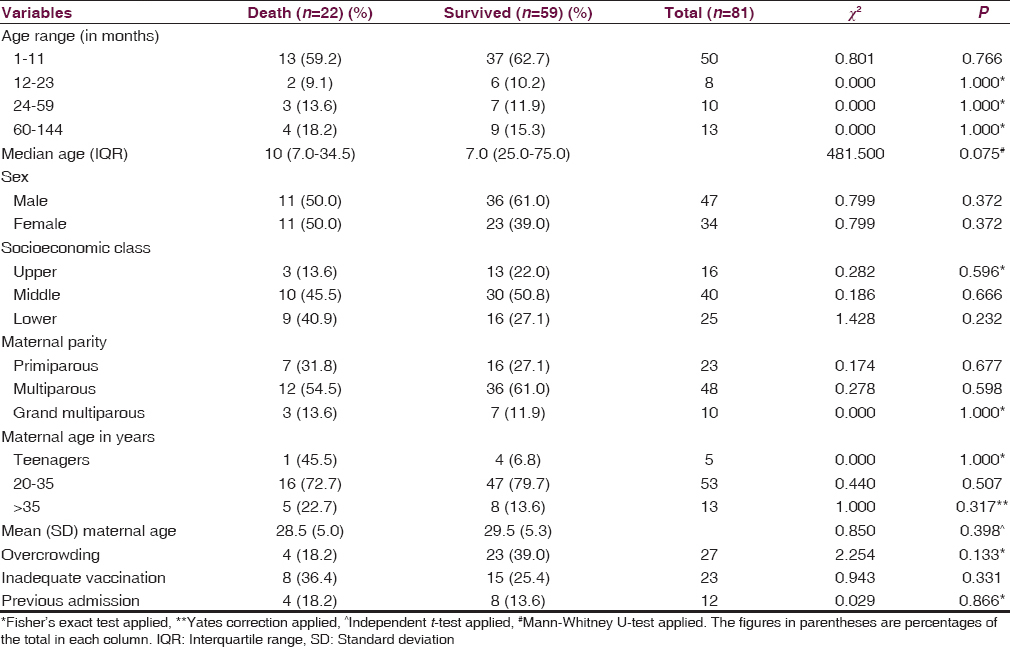
The median (IQR) ages of children with meningitis who died were not significantly different from the median (IQR) age of those who survive (10.0 [28] vs. 7.0 [20]; Mann–Whitney U = 481.50; P = 0.075). Although, a higher proportion of infants compared to other age groups died, the difference was not also significant (χ2 = 0.004; P = 0.949; df = 1) [Table 5].
Although, more proportion of children from middle and lower socioeconomic classes died compared to those from high socioeconomic class, the difference was not significant. No other sociodemographic factor among the children was significantly associated with mortality [Table 5].
Presenting features and mortality
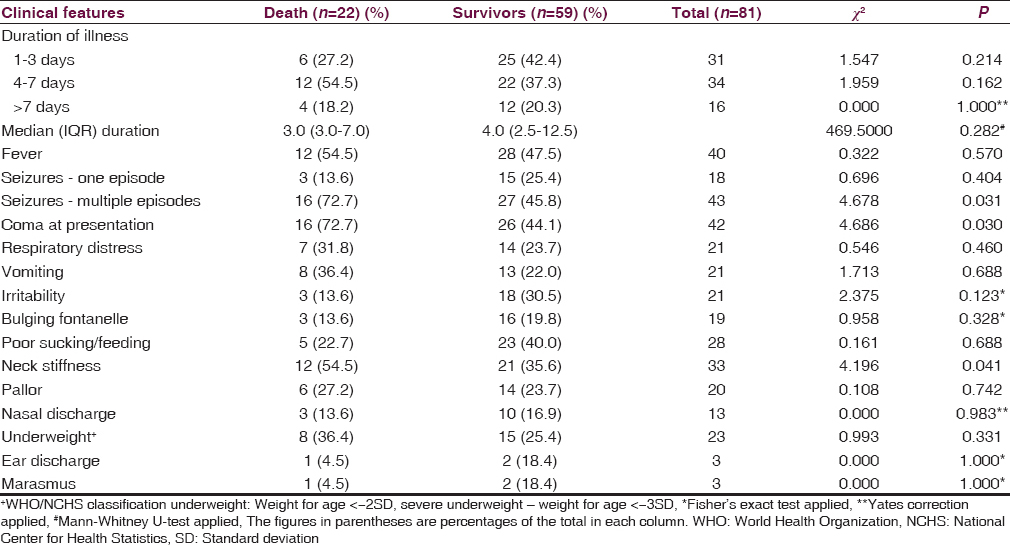
Children with bacterial meningitis who had multiple seizures at presentation were significantly more likely to die as 16 (37.2%) of the 43 children with multiple seizures compared to 6 (15.8%) of the remaining 38 children without multiple seizures, died (χ2 = 4.678; P = 0.031; df = 1). Similarly, coma (38.1% vs. 15.4%; χ2 = 4.686; P = 0.030) as well as neck stiffness/retraction at presentation (36.4% vs. 20.8%; χ2 = 4.196; P = 0.041) were significantly associated with mortality among these children [Table 6].
Laboratory findings and mortality
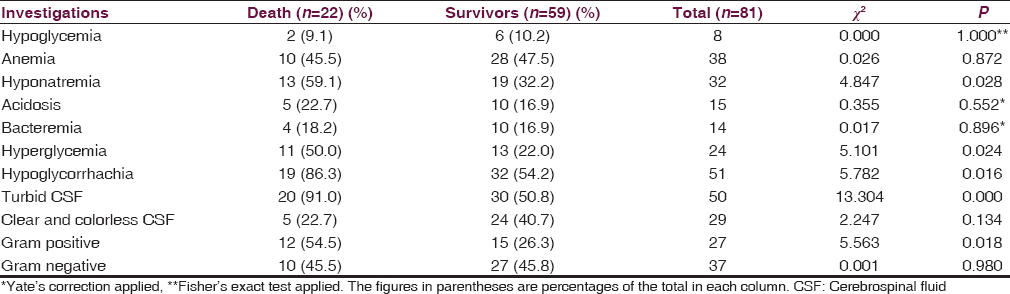
Children with hyponatremia at presentation were more likely to die from bacterial meningitis compare to those without hyponatremia (40.6% vs. 18.4%; χ2 = 4.847; P = 0.028; df = 1). Hyperglycemia at presentation was equally more frequently associated with mortality in childhood meningitis (45.8% vs. 19.3%; χ2 = 5.101; P = 0.024; df = 1). Likewise, low CSF glucose (P = 0.016), turbid CSF (P < 0.001) and the presence of Gram-positive yield on CSF Gram stain examination (P = 0.018) [Table 7].
Predictors of mortality in childhood pyogenic meningitis
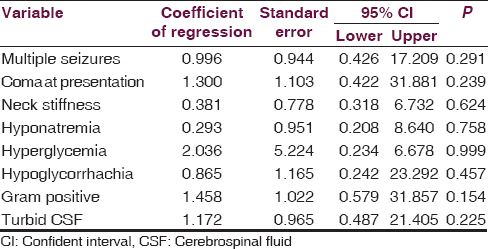
The variables found to be significantly associated with death among the children with meningitis using univariate analysis [Tables 5–7] were further subjected to multivariable regression analysis. None of the variables was found to independently predict death in children with bacterial meningitis [Table 8].
Discussion
The present study has presented data on the epidemiology, clinical presentations, and determinants of mortality in childhood bacterial meningitis from a tertiary center in Southwest Nigeria. The incidence of 5.5% observed among hospitalized children in the present study was higher than 1.6% reported from the same center by Ogunlesi et al.[5] about 10 years earlier. This may be due to the displacement of quite a number of families from the meningitis belt of the Northern Nigeria to the South because of the unrest and terrorist insurgency over the years may contribute to this high incidence.[13] A global review on internal displacement in sub-Saharan Africa revealed that about 3.3 million persons have been internally displaced in Nigeria (in 2013). Majority of these displacements are due to terrorist attacks and insurgency.[14] These displaced persons mostly live in internally displaced camps in the country, while some live with relatives and friends in locations free from the terrorist attacks in the south of the country including Ilesa the site of this study.[14] Such displacement reduce the standard of living of the people involved, lead to overcrowding, and easy spread of infection.[1314]
The organisms isolated from the CSF of children with meningitis in this study revealed that about one-half of the isolates were H. influenzae followed by pneumococcus. These findings were not unexpected because there is poor routine childhood immunization coverage and vaccines against pneumococcus as well as Haemophilus were not part of routine immunization schedule in the country during the study period (2011–2013). The immunization coverage in Nigeria (in 2013) as measured by the number of children who had DPT3 was estimated to be 41%.[15] The need for improved routine immunization uptake to ensure child survival cannot be overemphasized.
The etiological agents isolated in this study are similar to the isolates reported from other centers in developing countries[678910] and from developed countries before the introduction of H. influenzae type B conjugate vaccine and pneumococcal conjugate vaccine.[2] There is, however, increasing resistance to chloramphenicol and ceftriaxone which used to have 95.0% and 100% respective sensitivity to the organisms 10 years ago.[5] All the organisms, however remains sensitive to ciprofloxacin. The indiscriminate use of antibiotics for childhood illnesses and high prevalence of preadmission use of antibiotics may be responsible for increasing resistance of the organisms to these antibiotics. Regular surveillance on antibiotic sensitivity pattern in childhood infection, and rationale use of antibiotics is hereby strongly advocated.
The 27.2% of mortality observed in this study is similar to 26.6% reported by Ogunlesi et al.[5] from the same center 10 years earlier and 26.8% reported by Lagunju et al.[7] from Ibadan. This is, however, lower than 5–10% mortality reported from developed countries.[1216] This may be related to late presentation of patients to the hospital and nonavailability of advance life support for patients in need of them in developing countries. Also, high proportion of survivors with neurologic deficits observed in this study is similar to reports from Yemen[17] but more than about 20% reported from developed countries.[18] This may be related to high proportion of infants in our study with relatively poor immunity and rapidly growing brain thus more likelihood to develop neurologic sequelae of infections involving the brain.
Multiple seizures at admission were found in the present study to be associated with mortality among the children with meningitis. Multiple seizures may occur from cerebral irritation caused by the inflammatory process; cerebral edema; and subdural effusions which often complicate meningitis in children may also be responsible for the seizures.[2] Convulsions may also result from electrolyte derangement and hypoglycemia. Also, herbal remedies and concoction often use in an attempt to treat a sick child in developing countries may also contribute to the seizures seen in children with meningitis.[19] The more the number of episodes and duration of convulsions, the more the likelihood of brain damage; hence the poor prognosis associated with multiple seizures.
Coma at presentation was found in the present study to be associated with mortality. This is in agreement with reports from previous studies.[617] Coma in childhood meningitis may result from cerebral hemorrhages or thrombosis, hypoglycemia or even cerebral edema.[220] The underlying pathologic process leading to the coma couple with poor monitoring and care given to comatose children due to inadequate monitoring facilities and or personnel may contribute to the increased mortality observed in comatose meningitis children.
The presence of hyponatremia in children with bacterial meningitis was associated with increased risk of mortality from the present study. Hyponatremia may be a sequelae of syndrome of inappropriate antidiuretic hormone secretion which is a recognized complication of central nervous system infections including meningitis.[220] It may also due to failure of the ATPase-dependent membrane Na+/K+ pump (sick cell syndrome) leading to net intracellular influx of sodium and water with subsequent cellular damage and death,[20] hence the poor prognosis observed among this group of children. This implies that children admitted with meningitis should be closely monitored for electrolyte derangement particularly serum sodium to improve their chances of surviving.
The CSF appearance in bacterial meningitis is a reflection of the invasion of the hitherto sterile CSF by bacterial agents. Changes in CSF morphology and biochemistry have been correlated with prognosis in various studies.[2820] In the present study low CSF glucose, turbid/cloudy CSF as well as Gram-positive yield on CSF Gram stain were significantly associated with mortality. These agree with report by Johnson et al.[8] in Ilorin Nigeria. Turbidity of the normally clear and colorless CSF is a reflection of the amount of pus (polymorphonuclear) cells in it which in turn is determined by the quantity and virulence of the invading organisms as well as the host immune response.[2] These cells thrive on the CSF glucose reflecting the low CSF glucose associated with this condition. Turbid CSF, therefore, reflects the invasion of the CSF by rapidly proliferating pathogenic agents with reactive polymorphonuclear pleocytosis hence the poor prognosis associated with it.
We appreciate the limitation that detection of etiologic agents causing bacterial meningitis in this study was limited to culture of the CSF. More sophisticated methods such as polymerase chain reaction and agglutination test for bacterial antigens to detect some etiologic agents causing childhood meningitis particularly in partially treated cases would have been more revealing but facilities for such are not available; likewise the neuroimaging, audiometric, and psychometric assessments of survivors which were not done in this study may also have been increased the incidence of neurologic deficits among survivors as some with subtle deficits could have been detected.
Conclusion
Bacterial meningitis continue to be a major cause of morbidity and mortality with infants most susceptible. The causative organisms remain largely unchanged from previous reports but the antibiotics sensitivity pattern revealed increasing resistance to commonly used antibiotic agents. Multiple seizures, neck retraction, and coma at presentation as well as hyponatremia, hyperglycemia, and turbid CSF increase the risk of death in childhood meningitis. None of these, however, independently predicts mortality in the children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors hereby acknowledge the contributions of all the clinicians, nurses and others who participated in the care of the children as well as the laboratory scientists who helped with the analysis of the samples.
References
- Global burden of disease and injuries series. Global Health Statistics. Vol II. Geneva: World Health Organization; 1996. p. :285.
- [Google Scholar]
- Central nervous system infections. In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson's Textbook of Pediatrics (18th ed). Pennsylvania: WB Saunders Company; 2007. p. :2038-44.
- [Google Scholar]
- Diagnostic accuracy of clinical symptoms and signs in children with meningitis. Pediatr Emerg Care. 2011;3:196-9.
- [Google Scholar]
- Social ramifications of control of microbial disease. Johns Hopkins Med J. 1982;151:302-12.
- [Google Scholar]
- Childhood bacterial meningitis beyond the neonatal period in southern Nigeria: Changes in organisms/antibiotic susceptibility. East Afr Med J. 1994;71:14-20.
- [Google Scholar]
- Childhood bacterial meningitis in Ibadan, Nigeria – Antibiotic sensitivity pattern of pathogens, prognostic indices and outcome. Afr J Med Med Sci. 2008;37:185-91.
- [Google Scholar]
- Bacterial pathogens and outcome determinants of childhood pyogenic meningitis in Ilorin, Nigeria. Afr J Med Med Sci. 2001;30:295-303.
- [Google Scholar]
- Bacteriology and sensitivity patterns of pyogenic meningitis at Kenyatta National Hospital, Nairobi, Kenya. East Afr Med J. 1995;72:658-60.
- [Google Scholar]
- Ilesa West Local Government Area. Available from: http://www.info@ilesawestlg.os.gov.ng
- [Google Scholar]
- Socioeconomic and cultural background of hospitalized children in Ilesa. Niger J Paediatr. 1985;13:111-8.
- [Google Scholar]
- The Boko Haram uprising and Islamic revivalism in Nigeria. Afr Spectr. 2010;45:95-108.
- [Google Scholar]
- Internal Displacement in Sub-Saharan Africa. Global Overview 2014, Norwegian Refugee Council and Internal Displacement Monitoring Centre. Available from: http://www.internal-displacement.org
- [Google Scholar]
- UNICEF. 2014. State of the World's Children. New York: United Nations Children's Fund; Available from: http://www.unicef.org
- [Google Scholar]
- Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970-6.
- [Google Scholar]
- Bacterial profile and clinical outcome of childhood meningitis in rural Yemen: A 2-year hospital-based study. J Infect. 2006;53:228-34.
- [Google Scholar]
- Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317-28.
- [Google Scholar]
- Acute bacterial meningitis beyond the neonatal period. In: Long S, ed. Principles and Practice of Pediatric Infectious Diseases Revised Reprint (3rd ed). Philadelphia, Pa, USA: Churchill Livingstone; 2008. p. :284-91.
- [Google Scholar]






