Translate this page into:
Efficacy and Tolerability of Lacosamide in Lennox–Gastaut Syndrome: A Systematic Review and Meta-analysis
Indar Kumar Sharawat, DM Division of Pediatric Neurology, Department of Pediatrics, All India Institute of Medical Sciences Rishikesh 249203, Uttarakhand India sherawatdrindar@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Purpose Lennox–Gastaut syndrome (LGS) is one of the most difficult to treat childhood-onset epileptic encephalopathies. There is growing evidence that lacosamide is safe and efficacious in patients and adults with refractory epilepsy. However, the evidence regarding the efficacy of lacosamide in LGS is controversial so far. We aimed to evaluate the efficacy and tolerability of lacosamide in patients with LGS.
Methods We conducted a systematic review on MEDLINE, EMBASE, COCHRANE CENTRAL, Google Scholar, and Web of Science, collating all available literature till July 31, 2020. The qualitative review included case reports, case series, and both controlled/uncontrolled trials as well as retrospective studies, but for determining pooled estimates, we only included studies with a sample size of 5 or more. The primary outcome was the efficacy of lacosamide in patients with LGS. Clinical variables related to efficacy and adverse events attributed to lacosamide were extracted from each publication. The pooled estimate of variables related to these parameters was performed using a random-effect model.
Results Of the 68 items identified by the search, 14 were reviewed as full-text. Eleven articles including two prospective and six retrospective studies fulfilled eligibility criteria and described outcomes in 81 patients (42 adults, 39 children, 60% male, range—1.4–61 years). On average, 35.2%, 27.9%, 7.3%, and 29.4% patients had > 50% reduction, < 50% reduction, no change, and worsening of seizure frequency, respectively. Although 36% of patients had adverse events like somnolence, behavioral abnormalities including irritability, aggressiveness, nausea, tremor, memory problems, dizziness, gastrointestinal discomfort, vomiting, and weight loss, no serious adverse events were noted.
Conclusion The evidence available in the current literature is not sufficient to support or refute the use of lacosamide in patients with LGS. Although it is one of the possible therapeutic options worth exploring in patients with LGS, caution is still necessary, as there are reports of worsening of seizure frequency in some patients.
Keywords
lennox–gastaut syndrome
epileptic encephalopathy
drug-refractory epilepsy
lacosamide
childhood-onset epilepsy syndrome
Introduction
Lennox–Gastaut syndrome (LGS) is a childhood-onset epilepsy syndrome, which often becomes resistant to antiepileptic drugs and is almost always associated with intellectual deterioration after seizure onset, learning disability, and/or behavioral difficulties as comorbidities.1 It comprises 3 to 10% of all epilepsies in childhood, with the usual age of onset between 3 to 6 years. It is characterized by multiple seizure types like focal and generalized tonic seizures, atonic, myoclonic, and atypical absence seizures.1 Tonic seizures occurring during sleep are considered one of the characteristic features in patients with LGS. Diffuse generalized slow-spike wave (1.5–2.5 Hz) or polyspike wave discharges during wakefulness and generalized paroxysmal fast activity in nonrapid eye movement sleep (bursts of generalized fast rhythmic [8–26 Hz]) discharges, with frontal predominance, lasting at least for 2 seconds) are characteristic EEG findings.1 Valproate, levetiracetam, benzodiazepines, and topiramate are often used in patients with LGS. Still, the seizures remain uncontrolled in the majority of these patients and require dietary therapy, vagal nerve stimulation, and epilepsy surgery. In recent years, rufinamide and stiripentol are also being explored in these patients with favorable results.2 3 Lacosamide, one of the latest antiepileptic drugs, with sodium channel blocking properties is especially useful in refractory focal epilepsy in patients and adults. Its use in LGS has been controversial, with some reports suggesting favorable clinical response, while some other reports described the worsening of seizures in LGS with the use of lacosamide.4 5 6 7 This systematic review has been designed to assess the efficacy and safety of lacosamide in patients with LGS.
Methods
Search Methods
We performed a systematic review of the evidence on efficacy, safety, and tolerability of lacosamide in patients with LGS from the currently available literature. Accordingly, the primary objective of this systematic review was to provide a pooled estimate of the efficacy of lacosamide in patients with LGS in terms of total number seizure reduction (proportion of patients with > 50% seizure reduction). The secondary objectives were to provide a pooled estimate of the proportion of patients with worsening of seizure frequency and other adverse effects after initiating lacosamide. The review also intended to determine the efficacy of lacosamide for reducing the frequency of individual seizure types seen in LGS, such as tonic, atonic, myoclonic, tonic-clonic, and atypical absence seizures.
A meta-analysis of observational studies in epidemiology (MOOSE) and Recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was followed while reporting this systematic review and meta-analysis. A predefined search strategy was initially developed. The systematic review was registered with international prospective register of systematic reviews (PROSPERO).
Two authors independently performed a systematic literature search (on August 1, 2020) for all articles published till July 31, 2020, on “MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Google Scholar, and Web of Science databases.” We used the following keywords: “lacosamide,” Lennox–Gastaut Syndrome,” “patients,” “adults,” “child,” “childhood,” “focal epilepsy, “generalized epilepsy,” “drug-resistant epilepsy,” “refractory epilepsy,” and “seizures.” Bibliographies of pertinent reviews, all other searched items, and relevant conference proceedings were searched to find additional documents. When necessary, the study authors were contacted by e-mail for additional information not mentioned in the published article. We also sought additional studies by searching the Internet for ongoing trial registers (clinicaltrials.gov) with preliminary published results.
Eligibility of Studies
As there were only a few studies were exploring the efficacy of lacosamide in patients with LGS, hence uncontrolled/controlled clinical trials, prospective cohort studies, retrospective studies, case series, and case reports were also included in the qualitative review. For a quantitative review of the results, we included studies with at least five cases of LGS, who received lacosamide, to obtain a more accurate pooled estimate of its efficacy. However, all attempts were made to retrieve individual patient data (IPD) to avoid bias and perform an IPD systematic review.
Only studies which assessed the efficacy of lacosamide in patients with LGS or enrolled at least one patient with LGS as part of a large study were included in the review irrespective of the language or country of publication. If any study was found to have multiple publications, all versions of that particular study were reviewed to obtain complete access to maximal data from the study. Even brief abstracts/published conference proceedings and mixed population cohort studies enrolling both patients and adults were enrolled in the review if adequate data were available in the publication. However, duplicate entries and publications enrolling repeated populations were excluded.
Study Selection, Data Extraction, and Assessment of the Risk of Bias
All selected eligible articles were subjected to full-text review by two independent authors. They also evaluated the methodological quality of documents. The relevant data that were extracted after full-text review from the included articles are the following clinical and outcome variables: study design; study period; sample population; number of patients; seizure type (tonic, clonic, tonic-clonic, myoclonic, atonic, atypical absence); frequency of seizures at baseline and after instituting lacosamide; follow-up duration; the proportion of patients with > 50% and < 50% reduction in seizure frequency; the proportion of patients with unchanged seizure frequency, complete seizure freedom, or worsening of seizures; the number of patients with worsening of electrographic pattern; the number of patients with status epilepticus (tonic or of other semiology); lacosamide dosing regimen: initial, maximum and median dose of lacosamide tried; type, frequency, and severity of adverse effects (somnolence, gait instability, behavioral worsening); and the number of patients who prematurely discontinued lacosamide due to adverse effect/ineffectiveness/worsening of seizure frequency. The data were transferred to a Microsoft Excel spreadsheet after uniform and systematical extraction of data in a standardized predetermined form. By mutual discussion, the review was resolved for any discrepancies regarding inclusion. To ensure the accuracy and completeness of the extracted data, another third independent investigator was asked to perform quality check for the extracted data. If some disagreement used to occur between both investigators on some topic, then the third investigator was involved in the discussion to achieve a consensus decision. To avoid duplication of data, all possible efforts were made. The cases which were not included previously as part of another series only were included in the final analysis.
All the included studies/series/case reports were a part of qualitative analysis of systematic review, but while subjecting to metanalysis for various parameters, we only included case studies/case series with at least five cases of LGS to obtain a more meaningful, unbiased, and relevant pooled estimate.
The Newcastle Ottawa scale was used to assess the quality of the included studies. The studies were classified as good, fair, and poor quality. The level of evidence and quality of recommendations mentioned in the included study was determined by two investigators independently, using GRADE's approach, and any dispute was settled by discussing with a third investigator. The risk of bias in the included studies was determined by the “Risk Of Bias In Nonrandomized Studies of Interventions (ROBINS-I)” tool.
The following scoring system was used: “good quality: at least 3/4 stars in selection domain, 1/2 stars in comparability domain, and 2/3 stars in outcome/exposure domain; fair quality: 2 stars in selection domain, ½ stars in comparability domain, 2/3 stars in outcome/exposure domain; and rest of the studies: poor-quality studies.” Similarly, the quality of case reports was checked according to CAse REport (CARE) guidelines, and case reports were classified into good, fair, and poor qualities, depending on whether they satisfied all 13 criteria, at least 10, or less than 10 criteria, respectively.
Outcome Measures
The primary efficacy outcomes were the proportions of patients who achieved < 50%, ≥ 50%, and 100% reduction in the overall frequency of all kinds of seizures during the treatment and maintenance periods with lacosamide in patients with LGS. ILAE 2017 recommendations were followed to describe the seizure and epilepsy terminologies to maintain uniformity in the review.
Secondary efficacy endpoints were the proportions of patients with worsening of seizure frequency as compared with baseline. The other secondary efficacy outcomes were the proportion of responders for individual seizure semiologies like atonic, tonic-clonic, or atonic seizures.
The safety outcomes were the proportion of patients experiencing any of the adverse events (AEs) reported to be commonly related to lacosamide, based on previous evidence, the proportion of patients with serious adverse events (SAEs), the proportion of patients with hematological, biochemical abnormalities or abnormalities in liver and renal function tests, the proportions of patients discontinuing lacosamide for AEs, ineffectiveness or any other reason, and the total number of patients discontinuing lacosamide prematurely. We also reviewed whether any study reported the variations in measures of motor or global functioning, sleep, quality of life, and behavioral adaptation of patients from baseline to the end of treatment in measures of global functioning, assessed by validated scales, Likert scales, or patient or caregiver global impression of change
Data Synthesis and Statistical Analysis
The categorical variables were expressed in frequency (in percentage) along with 95% confidence interval (CI), and the continuous variables were expressed as mean with standard deviation (SD) or median with interquartile range (IQR). Among the included variables in the review, the pooled estimate was determined along with upper and lower 95% CI, whenever it seemed feasible. Revman 5.4 software and SPSS statistical software package was used to compare the pooled estimate of these parameters, including a meta-analysis of data regarding various parameters. To assess heterogeneity in studies, Higgins and Thompson's I2 method and Cochran's Q statistics with Chi-square test were utilized. The presence of publication bias was assessed using Egger's test. When I2 was more than 50%, a random-effect model was utilized, and a fixed-effect model was utilized for the rest of the parameters.
Results
Results of the Search
After a primary search, a total of 68 publications were retrieved. Among these, 29 were duplicates and hence removed accordingly. The eligibility of the remaining 39 papers was evaluated initially, and 25 irrelevant articles were excluded according to the title, article type, and abstract (Fig. 1). Ultimately, 14 articles were selected for full-text review, out of which 11 were included in the systematic review.8 9 10 11 12 13 14 15 16 17 18 Out of these, two were prospective studies, six were retrospective studies, one was a prospective small case series of three cases, and two were single case reports. Seven articles described only the pediatric population and four articles described the adult population. Four studies were meant to determine the efficacy of lacosamide in refractory epilepsy of all etiology and only a proportion of patients were suffering from LGS.
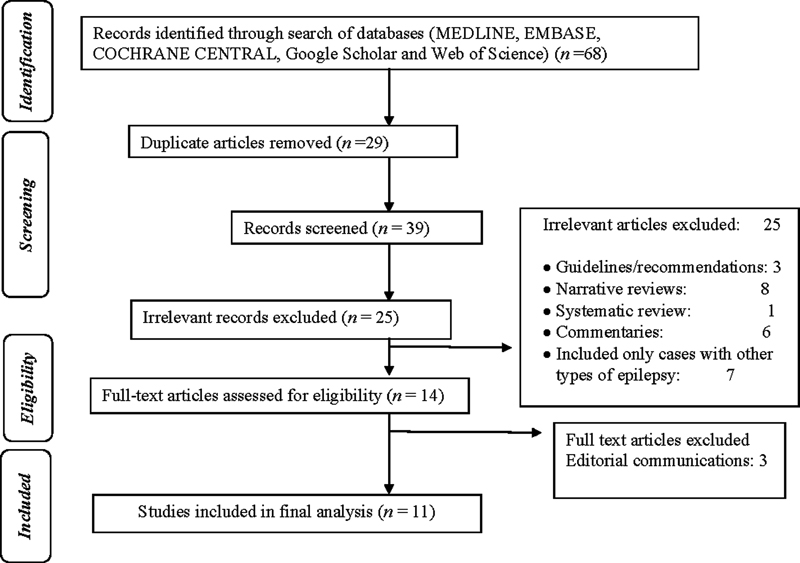
-
Fig. 1 Flow diagram of the study selection process.
Fig. 1 Flow diagram of the study selection process.
Characteristics and Risk of Bias of Included Studies
Out of the 9 studies, 6 were of good quality, 2 were of fair quality, and 1 was of poor quality. There was a moderate risk of bias in all these studies, according to the ROBINS-I tool. None of the studies were of poor quality. The certainty rating for the level of evidence provided in the studies was of the low level of evidence for 8 studies and a moderate level of evidence only for one study, as most of the studies had a small sample size and without any control group. Both case reports were of fair quality. No significant publication bias was found.
Qualitative Review
Grosso et al in a retrospective study demonstrated that 6 (33%) out of 18 patients (average age—12.3 years) had > 50% seizure reduction after a mean follow-up period of 9 months. The patients were already receiving a median of 4 antiepileptic drugs (AEDs) (range: 2–9 AEDS), suggesting that all of them had drug-resistant epilepsy. None of the responders achieved complete seizure freedom. One more child had a < 50% seizure reduction. Only three patients had a worsening of seizure frequency (all had symptomatic LGS), while the rest eight patients had an unchanged seizure. Mean dose of lacosamide tried in this study was 15.2 mg/kg/day (range: 9.8–18.1 mg/kg/day).10
While the overall seizure reduction rate from baseline was 29%, the same for tonic seizures and drop attacks was 31% and 20%, respectively. However, three out of four patients with focal tonic seizures had > 50% reduction in seizure frequency. Around 44% of the participants suffered from some kind of adverse effects. Nausea, vomiting, and dizziness were the most common adverse effects. But none of the adverse effects led to permanent health problems. Four patients discontinued (3 because of ineffectiveness and one because of walking instability). None of the participants had any laboratory abnormalities in routine hematological and biochemical parameters.10
Cuzzola et al described three young adults with LGS, who had an increase in tonic seizure frequency after starting lacosamide, and among them, one patient also had tonic status epilepticus. After discontinuing lacosamide, all three patients returned to baseline clinical status. All three of them were receiving four to five AEDs previously. The dose of lacosamide tried in them was 100 to 200 mg/day.14
Andrade-Machado et al retrospectively reviewed 19 adults with LGS, who have already received at least three AEDs, seven had undergone corpus callosotomy, and three also had vagal nerve stimulation therapy before the institution of lacosamide. The median dose of lacosamide was 200 mg (range-50–300 mg). Only two patients had a > 50% reduction in seizure frequency and one patient had a 25 to 50% reduction in seizure frequency. The rest of the 16 patients had a worsening of tonic seizures, while seven patients had a worsening of atonic seizures, but none had a worsening of tonic-clonic seizures. The highest seizure reduction was observed for patients with focal seizures and tonic-clonic seizures (13/19). Those with worsening of seizure frequency reached a clinical baseline after stopping the drug. Cryptogenic or symptomatic etiology had no significant statistical association with a reduction in the frequency of the various type of seizures (atonic, tonic, or tonic-clonic) or the number of patients with a repetitive cluster of tonic seizures or tonic status. Nevertheless, the response rate for all seizure types was higher in patients with symptomatic etiology. All patients who had > 25% reduction in seizure frequency were males, with a structural abnormality in magnetic resonance imaging (MRI) brain and focal discharges in EEG. Nine patients had behavioral abnormalities and other AEs (somnolence), but none of them had any laboratory abnormalities in routine hematological and biochemical parameters.9
Bermejo et al in a retrospective chart review on adults with LGS showed that 6/18 (33%) patients had > 50% and seven (38%) had < 50% reduction in seizure frequency after starting lacosamide. All of them were young adults with an average age of 23.3 years and the average follow-up period was around 6 months. None of them had worsening of seizures, but two patients discontinued due to ineffectiveness. Only three patients (17%) had some adverse effects. In this study, there was no significant difference across subgroups.16
Miskin et al performed a retrospective study on 21 patients (mean age—11.9 years) with refractory generalized epilepsy, out of which eight patients had LGS. Seven patients had a > 50% reduction in seizure frequency, while only one child did not respond. While average starting dose was 2.9 mg/kg/day (0.8–5.9 mg/kg/day), average maintenance dose was 6.9 mg/kg/day (1.7 to 14.3 mg/kg/day). The average follow-up duration was 19 months, longer than other studies. A significant proportion of these patients had also failed the ketogenic diet and vagal nerve stimulation (VNS) previously. Only 19% received lacosamide as monotherapy, while the rest 81% received it as an adjunct therapy. Around 28% of patients had some or other adverse effects.17
A similar retrospective study performed by Yorns et al included 40 patients with refractory epilepsy, out of which 5 patients were suffering from LGS. While three patients had a > 50% reduction in seizure frequency, one child had a < 50% reduction in seizure frequency and one child had a worsening of seizure frequency. The average follow-up duration was 9.2 months, and the average maintenance dose was 7.04 ± 4.23 years.17
Rastogi et al have described four patients (2 boys, 2 girls, average age 9.75 years) with LGS, who have previously failed five to nine AEDs, as part of a prospective study in patients with refractory epilepsy. Two patients had a > 90% reduction in seizure frequency, while the rest of the two patients had unchanged seizure frequency. The average length of follow-up was 9.8 months and the average dose of lacosamide tried in this study was 9.4 mg/kg/day. Adverse effects reported were nausea, vomiting, gastrointestinal intolerance, dizziness, headaches, and somnolence.12
Casas-Fernandez et al have described two patients with LGS as part of a large prospective observational study in Spain, both of whom had > 50% reduction in seizure frequency after instituting lacosamide at a dose 6.80 ± 2.39 mg/kg/day for 3 months.15
Heyman et al have described two toddlers (2 years and 1.5 years) with LGS as a part of a large retrospective study, who had exhausted the option of five to six AEDs, pyridoxine, and ketogenic diet, increased up to a dose of 18 to 20 mg/kg/day, but still had worsening of seizure frequency. One child had some improvement in motor function (probably unrelated to the AED effect), but the other child had restlessness and excessive crying, apart from nausea. They did not mention whether the seizure frequency reached baseline after discontinuing lacosamide.13
Algahtani et al have described a 22-year-old female with LGS resistant to multiple AEDs, who had a > 50% reduction in the frequency of tonic, clonic, and tonic-clonic seizures on lacosamide, but developed an unusual side effect of excessive laughing (not gelastic seizures). After discontinuing lacosamide, the seizure frequency worsened again, hence lacosamide was reconstituted and excessive laughing recurred. But the parents were reassured to continue lacosamide.8
Andrade Machado et al have previously described a 20-year-old male with LGS, who had failed at least nine AEDs previously, had worsening of tonic seizures and electroencephalographic pattern after starting lacosamide, and after discontinuing the drug, the patient returned to clinical and electrical baseline.11
Quantitative Review
The 11 articles described a total of 81 patients (42 adults, 39 patients, 60% male, range—1.4–61 years). The median number of AEDs already tried in these patients was 5 (IQR—3–7). A total of 31 (39%) patients had also received one of the nonpharmacological options: ketogenic diet, VNS, or epilepsy surgery. Out of these 81 patients, 29 (35%), 19 (23%), 7 (8.6%) and 26 (32%) patients had > 50% reduction, < 50% reduction, unchanged seizure status, and worsening of seizure frequency, while 22/60 (36.6%) patients experienced at least one adverse effect (Table 1).
|
Author |
Type of study |
Sample size |
Sample population |
M:F |
No. of patients with > 50% reduction in seizure frequency |
No. of patients with < 50% reduction in seizure frequency |
No. of Patients with unchanged seizure frequency |
No. of Patients with worsening of seizure frequency |
Patients with adverse effects |
|---|---|---|---|---|---|---|---|---|---|
|
Grosso et al10 |
Retrospective |
18 |
12.3 ± 2.4 (5.6–15) |
12:6 |
6 |
9 |
0 |
3 |
8 |
|
Andrade-Machado et al11 |
Retrospective |
19 |
27.1 ± 14.2 (18–61) |
12:7 |
2 |
1 |
0 |
16 |
9 (47%) |
|
Bermejo et al |
Retrospective |
18 |
23.3 ± 3.0 (18–27) |
11:7 |
6 |
7 |
5 |
0 |
3 |
|
Miskin et al17 |
Retrospective |
8 |
11.9 (4–19.5) |
4:4 |
7 |
1 |
0 |
0 |
Overall, 6/21 (28%) |
|
Yorns et al |
Retrospective |
5 |
12.3 ± 4.8 (1.4–20.8) |
− |
3 |
1 |
0 |
1 |
Overall, 15/40 (37.5%) |
|
Rastogi et al12 |
Prospective |
4 |
5–13 |
2:2 |
2 |
0 |
2 |
0 |
NA |
|
Casas-Fernandez et al15 |
Prospective |
2 |
− |
− |
2 |
0 |
0 |
0 |
NA |
|
Heyman et al13 |
Retrospective |
2 |
1.5–2 |
2:0 |
0 |
0 |
0 |
2 |
NA |
|
Cuzzola et al14 |
Case series |
3 |
24–27 |
1:2 |
0 |
0 |
0 |
3 |
2 |
|
Andrade-Machado et al5 |
Case report |
1 |
20 |
1:0 |
0 |
0 |
0 |
1 |
0 |
|
Algahtani et al8 |
Case report |
1 |
22 |
0:1 |
1 |
0 |
0 |
0 |
0 |
|
Total |
− |
81 |
− |
45:29 |
29 |
19 |
7 |
26 |
22/60 |
Abbreviation: LGS, Lennox–Gastaut syndrome.
However, to obtain the true weighed pooled estimate of these parameters, we included only studies with sample size of 5 or more, and we found only 5 such studies with 68 participants. The following meta-analysis/pooled estimate results included only these participants. Out of these, 35.2% (95% CI—24.0–46.3%) and 27.9% (95% CI—17.7–38.0%) patients had > 50% reduction and < 50% reduction in total seizure frequency as compared with baseline after starting lacosamide (Figs. 2 and 3), while 7.3% (95% CI—2.4–16.3%) and 29.4% (95% CI—18.9–41.7%) patients had unchanged seizure frequency and worsening of seizure frequency (Table 2). Similarly, 5/33 (15.1%, 95% CI—5.1–31.9%), 11/33(33.3%, 95% CI—17.9–51.8%) and 17/33 (51.5%, 95% CI—33.5–69.2%) with tonic seizures had > 50% reduction, < 50% reduction and worsening of seizure frequency. For patients with atonic seizures, 3/29 (10.3%, 95% CI—2.1–27.3%), 17/29 (58.6%, 95% CI—38.9–76.4%) and 9/29 (31.0%, 95% CI—15.2–50.8%) had > 50% reduction, < 50% reduction and worsening of seizure frequency. Finally, for patients with tonic-clonic seizures, 13/21 (61.9%, 95% CI—38.4–81.8%), 6/21 (28.5%, 95% CI—11.2–52.1%) and 2/21 (9.5%, 95% CI—1.1–30.8%) had > 50% reduction, < 50% reduction and worsening of seizure frequency (Table 3). For atypical absence seizures, the calculation of pooled estimate was not possible due to unavailability of adequate information in the included articles. None of the patients achieved complete seizure freedom. The difference between response rate for various type of seizure semiologies were significant (p = 0.04), with the response being better for tonic-clonic seizures.
|
Variables |
No. of studies (number of participants) |
No of patients with the affected variable |
Pooled estimates % (95% CI) |
Heterogeneity (I2%) |
p-Value for I2 |
|---|---|---|---|---|---|
|
Overall > 50% reduction in seizure frequency with lacosamide |
5(68) |
24 |
35.2 (24.0–46.3) |
32 |
0.04 |
|
Overall < 50% reduction in seizure frequency with lacosamide |
5(68) |
19 |
27.9 (17.7–38.0) |
37 |
0.02 |
|
Worsening of seizure frequency with lacosamide |
5(68) |
20 |
29.4 (18.9–41.7) |
51 |
0.001 |
|
Patients with any adverse effect |
3(55) |
20 |
36.3 (23.8–50.4) |
35 |
0.03 |
Abbreviation: LGS, Lennox–Gastaut syndrome.
|
Author |
Sample size |
> 50% reduction in tonic seizure frequency |
< 50% reduction in seizure frequency |
Worsening of tonic seizure frequency |
> 50% reduction in atonic seizure frequency |
< 50% reduction in atonic seizure frequency |
Worsening of atonic seizure frequency |
> 50% reduction in tonic-clonic seizure frequency |
< 50% reduction in tonic-clonic seizure frequency |
Worsening of tonic-clonic seizure frequency |
|---|---|---|---|---|---|---|---|---|---|---|
|
Grosso et al10 |
18 |
4/14 |
8/14 |
2/14 |
3/10 |
5/10 |
2/10 |
2/6 |
2/6 |
2/6 |
|
Andrade-Machado et al11 |
19 |
1 |
3 |
15 |
0 |
12 |
7 |
11/15 |
4/15 |
0 |
|
Total |
37 |
5/33 |
11/33 |
17/33 |
3/29 |
17/29 |
9/29 |
13/21 |
6/21 |
2/21 |
Abbreviation: LGS, Lennox–Gastaut syndrome.
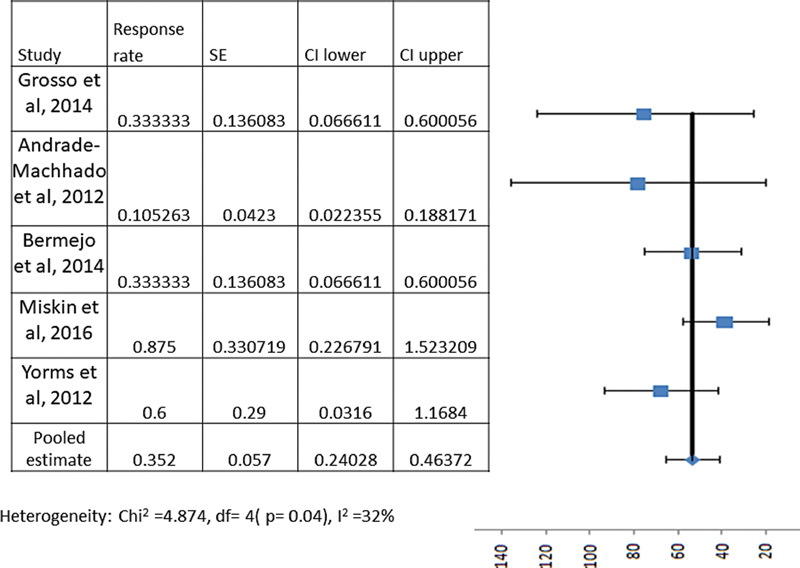
-
Fig. 2 Meta-analysis forest plot showing pooled estimate for the efficacy of lacosamide in patients with Lennox–Gastaut syndrome in terms of the number of patients with at least 50% reduction in seizure frequency.
Fig. 2 Meta-analysis forest plot showing pooled estimate for the efficacy of lacosamide in patients with Lennox–Gastaut syndrome in terms of the number of patients with at least 50% reduction in seizure frequency.
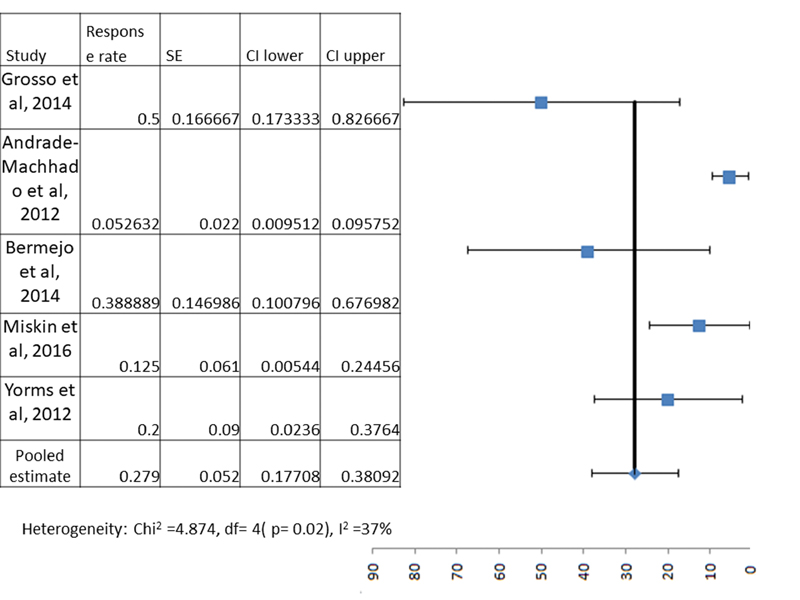
-
Fig. 3 Meta-analysis forest plot showing pooled estimate for the efficacy of lacosamide in patients with Lennox–Gastaut syndrome in terms of the number of patients with a < 50% reduction in seizure frequency.
Fig. 3 Meta-analysis forest plot showing pooled estimate for the efficacy of lacosamide in patients with Lennox–Gastaut syndrome in terms of the number of patients with a < 50% reduction in seizure frequency.
Subsequently, we performed a certain subgroup analysis to determine which group of patients is likely to respond better to lacosamide. Most of the patients were tried on lacosamide in the included studies after almost all the therapeutic options including dietary therapy, VNS, and epilepsy surgeries were exhausted. Only in 17% of patients, lacosamide was used early, that is, as a first or second add-on. Still, the proportion of patients with at least 50% reduction in seizure frequency was higher in this subgroup, compared with those in which lacosamide was tried as a late resort (47% vs. 33%, p = 0.04). While comparing for the percentage of patients in pediatric (< 14 years of age) and adult LGS group, who had ≥ 50%, < 50% response, no response, and worsening of seizure frequency, no significant difference was found, although there was a slight trend toward more favorable response in pediatric age group (p = 0.49). But it could be confounded by the fact that the number of previously tried antiseizure medications (ASMs), use of callosotomy and VNS were higher in adult patients, they had a longer epilepsy duration, and almost all of them received lacosamide as a last resort.
Then, we checked for the number of previous medications and comedications in those LGS patients who had at least some reduction in seizure and those who had worsening of seizure. The median number of medications previously tried were similar in both groups (5, IQR: 3–7). While valproate was the most common coadministered ASM in the subgroup with at least some improvement, levetiracetam was the most common in those with worsening of the seizures (but only minimally higher than the use of valproate). Overall, we could not find any particular difference between the combination of ASM therapy tried in these two subgroups, apart from the fact that three patients who had worsening of seizures, had lamotrigine as a comedication (also a sodium channel blocker), but none in the other subgroup had lamotrigine as a comedication (although some of them previously received lamotrigine). As lacosamide and lamotrigine both are sodium channel blockers, there might be some interaction between them, but future studies are required before reaching any firm conclusion in this regard. Topiramate, clobazam, zonisamide, and vigabatrin were other comedications in these patients, but we could not identify any particular ASM which has a definite synergistic effect with lacosamide. The proportion of patients with structural etiology was numerically higher in the subgroup with worsening of seizure, as compared with the counterpart, but it did not reach the point of statistical significance (41% vs. 36%, p = 0.72).
The initiating dose of lacosamide in all these studies was 2 to 3 mg/kg/day, while the maximum dose was 19 mg/kg/day (ceiling dose of 600 mg/day). The average dose in most studies ranged between 8 to 10 mg/kg/day. The duration for which lacosamide was tried in all these studies was at least 3 to 6 months, if the patient had immediate worsening of seizures, as in the case report by Andrade Machado et al with intravenous infusion of the drug.
Overall, 20/55 (36.3%, 95% CI—23.8–50.4%) patients experienced any kind of AEs after starting lacosamide, but none of them were SAE or life-threatening adverse effects. None of the patients experienced any kind of arrhythmia or abnormality in hematological or biochemical parameters and abnormality in liver/renal function tests. The AEs reported were somnolence, behavioral abnormalities including irritability, aggressiveness, nausea, tremor, memory problems, dizziness, gastrointestinal discomfort, vomiting, weight loss, and excessive laughing in one case.
Discussion
The current systematic review tried to addresses one of the controversial issues in the management of patients with LGS. The results suggest that although lacosamide can be successfully used to achieve seizure control in a particular subgroup of patients, especially with focal tonic or tonic-clonic seizures, caution is needed for each case. This is because a proportion of patients are likely to experience worsening of seizure frequency, electrographic pattern and, rarely, even tonic status epilepticus. However, the level of evidence behind this recommendation remains weak, as no controlled trials or uncontrolled trials with adequate sample size have been performed in this regard. Which subgroups of patients are likely to worsen with lacosamide is difficult to predict with currently available literature. This requires studies with a large sample size, which will correlate the change in seizure frequency with seizure and electrographic and pharmacogenomic profile of each patient.
Notably, most of the patients were tried lacosamide in the included studies after almost all the therapeutic options, including dietary therapy, VNS, and epilepsy surgeries, were exhausted. Thus, probably the sample population to start with represented those LGS patients, who are relatively more pharmaco-resistant than their counterparts. Thus, in the enrolled patients with any of the remaining therapeutic options, the chances of favorable seizure control might be already dismal. Future studies are required, which will explore lacosamide in patients with LGS early in the course of the disease in a placebo-controlled manner to determine the true efficacy of this drug. Lacosamide is as such an intriguing therapeutic option for most pharmaco-resistant epilepsies, due to its excellent safety profile, and thus deserves randomized control trials (RCTs) in patients with LGS also.19 In our review also, none of the patients were found to suffer from any serious or life-threatening adverse effects.
Moreover, the treatment of LGS to date is mainly based on physical experience, as controlled trials guiding clinicians in this regard are few in the literature.1 Although, it is typically resistant to commercially available AEDs, the sequence in which these AEDs tried is not clear and depends on the personal preference of clinicians.2
The exact pathogenesis as to why lacosamide caused the worsening of seizures in a few patients with LGS remains unknown. Phenytoin and carbamazepine, which are sodium channel blockers like lacosamide, often worsen myoclonic seizures in LGS.11 Thus, some authors attributed the sodium channel-blocking property of lacosamide to worsening of seizure frequency. However, lacosamide exerts its antiepileptic effects by a unique mechanism of increasing the slow inactivation of voltage-gated sodium channels, but it does not interfere with voltage-gated sodium channels with fast inactivation properties (targets for phenytoin and carbamazepine). As a result of this, an increasing number of sodium channels become unavailable for depolarization, important for the firing of action potentials of neurons and epileptogenesis.11
It also has an excellent first-pass oral bioavailability and favorable pharmacokinetic profile with only 15% protein binding. Unlike valproate, phenytoin, and carbamazepine, it has not been shown to interact with cytochromes P450 (CYPs) enzymes in clinical or preclinical studies.20 Thus, previously Andrade-Machado et al concluded that pharmacokinetic interaction does not appear to be the cause behind the worsening of seizures in LGS.11 Grosso et al opined that the small case series and case reports which documented lacosamide-induced worsening of tonic seizures might be incidental, and they could not really prove that the worsening is solely due to lacosamide.7 Italiano et al, on the other hand, speculated that the studies which have shown very favorable effects of lacosamide in LGS might be because tonic seizures in LGS are often subtle, nocturnal, and likely to be missed if the investigators rely totally on the caregivers' record.4 But, in our opinion, this fact applies to most of the drug RCTs in LGS and can be solved by an RCT only, where randomization will balance these confounding factors in both arms.
Finally, in other types of refractory epilepsies also, with both focal and generalized seizures, RCTs have shown that at least 30 to 40% of patients achieve > 50% seizure reduction with a dose of lacosamide at 400 to 600 mg/day.21 Novy et al in a large study showed that 45% out of 376 patients achieved long-term significant seizure control with lacosamide, and18 patients achieved complete seizure freedom; however, not specifically mentioned in that study was how many patients with LGS benefited from lacosamide.22
Most clinical studies and RCTs have also described relatively fewer and less severe adverse effects with lacosamide as compared with most of the traditional antiepileptic drugs. As often, the focal tonic seizures are considered the most difficult to control in LGS, thus theoretically lacosamide appears to be a reasonable choice for these patients.23 Even in the recent review on therapeutic options in LGS by Borreli et al, the authors concluded that several new antiepileptic drugs are available for LGS validated through RCTs and observational studies. But the results of studies exploring different drugs are difficult to compare, and thus they could not suggest one drug is more efficacious than others based on the available evidence.2
There are several limitations of this systematic review. All studies lacked a comparator arm, had a small sample size, and some of them only case reports/series. Thus, the reliability of the pooled estimate is questionable. But this is the first systematic review, to date, that attempted to provide a bird's eye view to clinicians on the efficacy and safety of lacosamide in LGS. Most studies were also retrospective studies and like other trials on LGS relied solely on caregiver records for seizure frequency. There was also significant selection bias in these study populations, as none of the studies explored lacosamide early in the course of LGS. Third, for some cases despite our best efforts, we could not get the individualized patient data, and thus the quantitative review was finally limited to five studies only.
Conclusions
Around 35% of patients with LGS achieve > 50% seizure reduction after lacosamide adjunctive therapy. But another one-third of patients may suffer from worsening of seizures. Thus, the evidence available in the current literature is not sufficient to support or refute the use of lacosamide in patients with LGS. Although, it is one of the possible therapeutic options worth exploring in patients with LGS, caution is still necessary, as there are reports of worsening of seizure frequency in some patients. RCTs are necessary to generate high-quality evidence favoring or disfavoring its use in LGS.
Conflict of Interest
None declared.
Funding None.
References
- Efficacy and safety of corpus callosotomy and ketogenic diet in children with Lennox Gastaut syndrome: a systematic review and meta-analysis. Childs Nerv Syst. 2021;37(8):2557-2566.
- [Google Scholar]
- Therapeutic approach to Lennox-Gastaut syndrome: a systematic review. Acta Neurol Belg. 2019;119(3):315-324.
- [Google Scholar]
- Efficacy and safety of rufinamide as adjunctive therapy in patients with Lennox Gastaut syndrome: a systematic review and Meta-analysis. Seizure. 2021;91:296-307.
- [Google Scholar]
- Use of lacosamide in Lennox-Gastaut syndrome: is it too premature? Acta Neurol Scand. 2014;130(2):e37-e38.
- [Google Scholar]
- Should be prescribed lacosamide in patients with Lennox-Gastaut syndrome? Acta Neurol Scand. 2014;130(2):e35-e36.
- [Google Scholar]
- Lacosamide in Lennox-Gastaut syndrome? Caution is still needed. J Child Neurol. 2016;31(14):1632.
- [Google Scholar]
- Efficacy and tolerability of add-on lacosamide in children with Lennox-Gastaut syndrome. Acta Neurol Scand. 2014;130(2):e39-e40.
- [Google Scholar]
- Lacosamide-induced excessive laughing in a patient with Lennox-Gastaut syndrome. Epilepsy Behav Case Rep. 2018;10:1-3.
- [Google Scholar]
- Efficacy and tolerability of add-on Lacosamide treatment in adults with Lennox-Gastaut syndrome: An observational study. Seizure. 2015;33:81-87.
- [Google Scholar]
- Efficacy and tolerability of add-on lacosamide in children with Lennox-Gastaut syndrome. Acta Neurol Scand. 2014;129(6):420-424.
- [Google Scholar]
- Lacosamide in Lennox-Gastaut syndrome: case report. Clin Neuropharmacol. 2012;35(3):148-149.
- [Google Scholar]
- Lacosamide in refractory mixed pediatric epilepsy: a prospective add-on study. J Child Neurol. 2012;27(4):492-495.
- [Google Scholar]
- Preliminary efficacy and safety of lacosamide in children with refractory epilepsy. Eur J Paediatr Neurol. 2012;16(1):15-19.
- [Google Scholar]
- Does lacosamide aggravate Lennox-Gastaut syndrome? Report on three consecutive cases. Epilepsy Behav. 2010;19(4):650-651.
- [Google Scholar]
- Efficacy and tolerability of lacosamide in the concomitant treatment of 130 patients under 16 years of age with refractory epilepsy: a prospective, open-label, observational, multicenter study in Spain. Drugs R D. 2012;12(4):187-197.
- [Google Scholar]
- Efficacy and tolerability of adjunctive lacosamide in pediatric patients with focal seizures. Neurology. 2019;93(12):e1212-e1226.
- [Google Scholar]
- Efficacy and tolerability of lacosamide in the treatment of children with refractory generalized epilepsy. J Child Neurol. 2016;31(7):925-928.
- [Google Scholar]
- Efficacy of lacosamide as adjunctive therapy in children with refractory epilepsy. J Child Neurol. 2014;29(1):23-27.
- [Google Scholar]
- Lacosamide: a review in focal-onset seizures in patients with epilepsy. CNS Drugs. 2018;32(5):473-484.
- [Google Scholar]
- Use of lacosamide in children with refractory epilepsy. J Pediatr Pharmacol Ther. 2012;17(3):211-219.
- [Google Scholar]
- Influence of adjunctive lacosamide in patients with seizures: a systematic review and meta-analysis. Int J Neurosci. 2018;128(7):670-676.
- [Google Scholar]
- Long-term retention of lacosamide in a large cohort of people with medically refractory epilepsy: a single centre evaluation. Epilepsy Res. 2013;106:250-256. (1-2):
- [Google Scholar]
- Efficacy of lacosamide and phenytoin in status epilepticus: A systematic review. Acta Neurol Scand 2021 (e-pub ahead of print)
- [CrossRef] [Google Scholar]






