Translate this page into:
Effectiveness of nurse-led fever, sugar-hyperglycemia, and swallowing bundle care on clinical outcome of patients with stroke at a tertiary care center: A randomized controlled trial
*Corresponding author: Lakshmi Ramamoorthy, Department of Medical Surgical Nursing, College of Nursing, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. laxmi_ramamoorthy@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Sridhar D, Ramamoorthy L, Narayan SK, Amalnath D, Lalthanthuami HT, Ganapathy S, et al. Effectiveness of nurse-led fever, sugar-hyperglycemia, and swallowing bundle care on clinical outcome of patients with stroke at a tertiary care center: A randomized controlled trial. J Neurosci Rural Pract. 2024;15:255-61. doi: 10.25259/JNRP_446_2023
Abstract
Objectives:
Stroke is a medical emergency, the leading cause of death, and a significant cause of disability in developing countries. The primary goals of stroke management focus on reducing disability, which needs prompt treatment in time. Fever, sugar-hyperglycemia, and swallowing (FeSS) bundle are a promising nurse-led composite for reducing disability and death. The present study aims to assess the effect of FeSS bundle care on disability, functional dependency, and death among acute stroke patients.
Materials and Methods:
A randomized controlled trial was conducted among 104 acute stroke patients, who were admitted within the first 48 h of stroke symptoms and had no previous neurological deficits. Randomization was stratified based on gender and type of stroke. The intervention group received FeSS bundle care, which included nurse-led fever and sugar management for the first 72 h, and a swallowing assessment done within the first 24 h or before the first oral meal. A follow-up assessment was done after 90 days to assess the disability, functional dependency, and mortality status using a modified Rankin scale and Barthel index.
Results:
No significant difference was noted in the 90-day disability and functional dependency between the groups. A reduction in mortality was noted in the intervention group. The risk ratio for mortality between groups was 2.143 (95% confidence interval: 0.953–4.820).
Conclusion:
Although no significant reduction in disability, there was a reduction in mortality in the intervention group. Hence, the study suggested the promotion of nurse-led intervention using the FeSS bundle in stroke units.
Keywords
Stroke
Acute stroke therapy
Patient monitoring
Activities of daily living
Survival analyses
Kaplan–Meier estimate
INTRODUCTION
Stroke, which occurs as a result of interrupted blood supply to the brain, remains the third leading cause of death and disability combined globally, and the stroke-related mortality rate is 3.6 times higher in lower-income countries.[1] The 30-day stroke case fatality rate in India varied from 41% to 42% in the urban population and 18–46% in the rural population.[2] Although improved stroke management in the past decades has led to decreased stroke mortality, still, it remains a very challenging problem in developing countries.[3] Chronic disability is found among 50% of stroke patients and disability risks are higher among the elderly population.[4]
Dedicated stroke units are proven to improve the patient’s condition and reduce mortality and morbidity through an enhanced team approach including focused monitoring and early rehabilitation by a specialized team.[5] However, stroke units are available only in major cities and managed mainly by private organizations, thus reducing access to most marginalized populations. Moreover, adherence to the practice of stroke care using the existing stroke guidelines often requires heavy resources, and therefore, often cannot be applicable in countries with insufficient resources where the emphasis has to be more on primary and secondary levels of the healthcare delivery system.
A care bundle to manage fever, sugar-hyperglycemia, and Swallowing (FeSS) which were actively led by nurses shows promising results in reducing death and disability for patients with stroke in another university-based hospital in Australia with a significantly high-risk reduction.[6] The FeSS bundle is considered standard care in many stroke units in developed countries.[7] Although audit and feedback programs advocate the efficacy of FeSS intervention to 10–14%, it is not practiced widely in Indian settings.[6]
The present study aimed to determine the effectiveness in reducing the mortality and disability rate by application of the FeSS bundle care in acute stroke patients after admission to the stroke care unit in an Indian setting using standard stroke assessment tools like the National Institutes of Health Stroke Scale.[8] The specific simple, effective evidence-based nurse-led FeSS bundle care, if found effective, will enhance the decision-making process for patients to help reduce the disability and mortality rate.
MATERIALS AND METHODS
A randomized controlled trial was conducted among acute stroke patients admitted to a public tertiary care center in South India.
Sampling and randomization
The sample size was calculated by anticipating a 20% difference in disability rate between the intervention and control group at a 5% level of significance and power of 80%; 52 patients were recruited in each group with an expected 10% attrition.[9] A convenient sampling technique was used to enroll the participants. Patients above 18 years of age, first-time stroke (ischemic or intracerebral hemorrhagic stroke), and who could speak Tamil or English were included in the study. Patients who arrived after 48 h of stroke and who did not stay in the unit for the first 72 h, patients suffering from a previous neurological deficit, palliative care patients, and cancer patients were excluded from the study.
The allocation sequence was generated by an independent statistician outside of the research team using permuted blocks of varying sizes. Randomization was stratified by covariates of gender (male and female) and type of stroke (ischemic and hemorrhagic stroke). Allocation concealment was done using sequentially numbered opaque sealed envelopes [Figure 1].
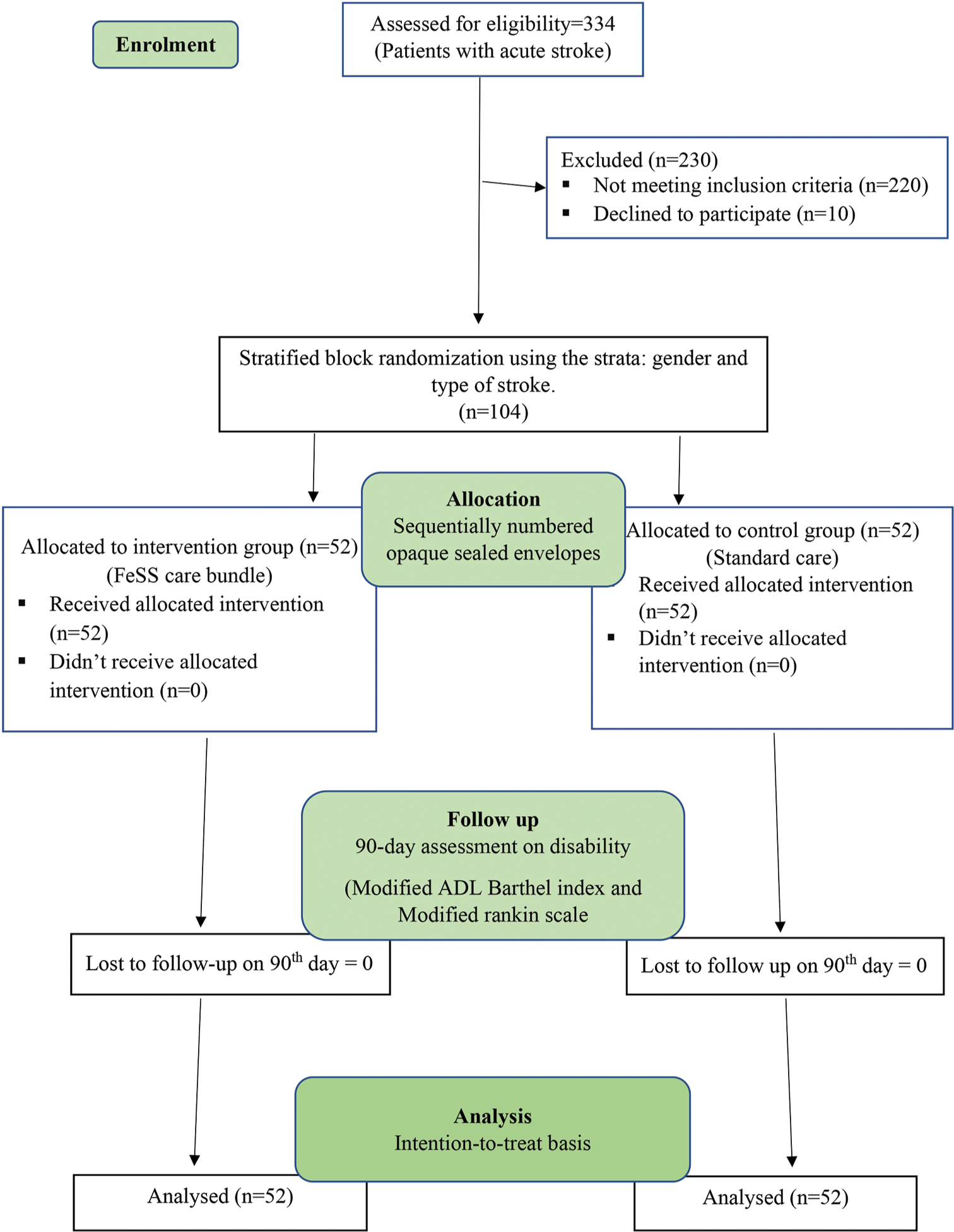
- Consort diagram, ADL: Activities of daily living. FeSS: Fever, sugar-hyperglycemia, and Swallowing, n: Number of patients.
Data collection instrument
The data collection pro forma consists of participants’ demographic and clinical characteristics, NIH stroke scale, FeSS protocol adherence, modified activities of daily living (ADL) Barthel index, and modified Rankin scale (mRS). The modified ADL Barthel index is a 10-item standardized tool widely used to measure physical disability relating to ADL among stroke patients. Validity and reliability were established by various studies with an internal consistency of 0.87–0.920.[10] The mRS is also a standardized tool commonly used to measure the composite outcome of stroke (Cronbach’s a of 0.86). The 6-item scale is used to determine the disability based on the range of activity performed by the patient.[11]
Data collection procedure
Before starting the study, a structured protocol and guidelines for the intervention group were framed, and nurses were trained. Datasheets for monitoring and assessment of the FeSS bundle were provided to respective stroke wards. The FeSS guidelines were displayed in respective stroke wards, and nurse champions in each ward were selected voluntarily. These nurse champions helped the researcher in coordinating the care of the study subjects, monitoring, and ensuring adherence to FeSS to allow blinding of the investigator for the follow-up assessment.
Participants were then concurrently enrolled and randomized into the control and intervention groups. Baseline demographic and clinical data including stroke severity were collected from both groups. The control group received standard care, i.e., temperature checking in every shift and as required, blood sugar monitoring once in emergency, and monitoring of blood sugar and administration of injection insulin as recommended by the treating physician, and oral feeding started after the instruction from the treating physician. For the intervention group, monitoring for fever, sugar, and swallowing was done as per the FESS bundle protocol.[12]
Fever monitoring
Patients were monitored for hyperthermia at baseline and every 4th h for the next 72 h. If the patient was febrile, then the temperature was monitored 2 hourly, and a paracetamol injection was administered within an hour. In the event of continuous fever or repeated fever, the treating physician was notified. The targeted temperature of 37.5°C was maintained in the intervention arm.
Sugar monitor and control protocol
The participants were monitored for hyperglycemia at baseline and 4th hourly for the next 72 h. If the patient was hyperglycemic, then capillary blood glucose was monitored 2 hourly and insulin was administered within an hour as per sliding scale under departmental policy. In the event of continuous hyperglycemia or repeated hyperglycemia, insulin infusion as per sliding sale was initiated, and the treating physician was notified.
Swallowing monitor and assessment protocol
Swallowing screen was performed within 24 h of admission or before starting orally in non-intubated patients. In the event of a nasogastric tube and intubation, the patient was deferred from swallowing screening. After a thorough explanation, the patient was placed in a 90° upright position and assessed for maintaining alertness for at least 20 min. If not, screening was terminated and planned for review.
The patient was assessed for facial weakness, drooping, slurred or absent speech, coughing on saliva, drooling, hoarse or absent voice, weak or absent cough, shortness of breath, and pre-existing swallowing difficulty.
The patient was then tested with a sip of water or ice cube, and observed for coughing, throat clearing, change in vocal quality, drooling, and change in respiration or shortness of breath. The same signs were assessed after providing a cup of water and after commencing a pre-morbid oral diet. The screening was terminated if any of the above were observed, and the treating physician was notified for speech pathologist consultation.
The 90-day follow-up assessment on disability (Modified ADL Barthel index and mRS) was collected either during their outpatient visit or through a telephone interview.
Statistical analysis
Data was analyzed using the Statistical Package for the Social Sciences (IBM SPSS Version 25.0). The distribution of categorical variables such as gender, comorbidity, and severity of stroke was expressed as frequency and percentage. Continuous variables such as age and pre-hospital delay were presented as mean with standard deviation and median with interquartile range. Distribution of data was checked using the Kolmogorov–Smirnov normality test. Comparison of baseline characteristics between groups was done using the Mann–Whitney test, Chi-square test, and Fisher’s exact test. A comparison of 90-day disability and functional dependency was conducted using the Chi-square test. Kaplan–Meier survival curves and log-rank test were used for assessing the difference in the distribution of time to event between the groups. Statistical analysis was performed on an intention-to-treat basis, at a 5% level of significance.
Ethical considerations
Permission was obtained from the Institute Nursing Research Committee (CON/NRMC/M.Sc./2020/MSN/1) and the Institute Ethics Committee for Human Studies (CON/ IEC/M.Sc/2020/MSN/1). The study was registered under the Clinical Trial Registry-India (CTRI/2021/11/038341). Ethical issues involved in the study were considered to be minimal risk as per ICMR guidelines. The procedures followed were by the ethical standards of the institution as well as the Declaration of Helsinki revised in 2013. Informed consent was taken from each participant’s parent or legally authorized representative voluntarily before enrolment as approved by the Ethics Committee. Participants were ensured of anonymity, confidentiality of their data, and the right to withdraw from the study before data collection.
RESULTS
The mean age of the participants was 51 (45, 65) and 50 (38.5, 56.0) years, respectively, in the control and interventional group. Both groups have an equal number of participants (50%) in terms of gender and type of stroke due to stratified randomization. There was no significant difference between the groups in terms of unit, comorbidity, and personal habits such as smoking, alcoholism, and tobacco usage. The groups were also homogenous in terms of pre-hospital delay and stroke severity (P > 0.05) [Table 1]. Six participants in the interventional group and four in the control group were allowed to take food orally. The need for insulin (39 vs. 12 times) and paracetamol (10 vs. 7) was higher in the intervention group.
| Variables | Intervention (n=52) n(%) | Control (n=52) n(%) | P-value |
|---|---|---|---|
| Age1 median (IQR) | 50 (38.5, 56.0) | 51 (45.0, 65.0) | 0.225 |
| Gender2 | |||
| Male | 26 (50) | 26 (50) | 1.000 |
| Female | 26 (50) | 26 (50) | |
| Type of stroke2 | |||
| Hemorrhagic | 26 (50) | 26 (50) | 1.000 |
| Ischemic | 26 (50) | 26 (50) | |
| Unit3 | |||
| Emergency | 38 (36.5) | 42 (40.3) | 0.587 |
| Intensive care unit | 9 (8.6) | 7 (6.7) | |
| Ward | 5 (4.8) | 3 (2.8) | |
| Comorbidity3 | |||
| None | 17 (16.3) | 17 (17.3) | 0.381 |
| Hypertension | 15 (14.4) | 15 (14.4) | |
| Diabetes | 6 (5.8) | 2 (1.9) | |
| Diabetes and hypertension | 6 (5.8) | 8 (7.7) | |
| Diabetes, hypertension, and others | 0 | 3 (2.9) | |
| Diabetes and others | 1 (1.0) | 0 | |
| Others (seizures, chronic kidney disease, rheumatic heart disease, and hypothyroidism) | 7 (6.7) | 6 (5.8) | |
| NIHSS severity of stroke2 | |||
| Minor stroke (1–4) | 3 (2.8) | 0 | 0.381 |
| Moderate stroke (5–15) | 23 (22.1) | 18 (17.3) | |
| Moderate to severe (16–20) | 12 (11.5) | 14 (13.5) | |
| Severe stroke (21–42) | 14 (13.5) | 20 (19.2) | |
| Pre-hospital delay (in minutes) median (IQR) | 430 (151.25,997.50) | 487.50 (258.5,970.50) | 0.333 |
| Duration of hospital stay (in days) median (IQR) | 11 (8, 17.75) | 9 (6.25, 13.75) | 0.060 |
n: Frequency, %: Percentage, IQR: Interquartile range, NIHSS: National institutes of health stroke scale, 1Mann–Whitney U-test, 2Chi-square test, 3Fisher’s exact test, *P≤0.05
Comparison of the mRS 90-day disability outcome between the intervention and control group shows an equal number of participants without dysfunction (23.1%). Nearly two-third of participants (63.4%) in the intervention group have dysfunction, whereas control groups have a lower (48.1%) number of participants with dysfunction. The number of deaths in the control group was higher as compared to the intervention group (28.8% vs. 13.5%). However, the difference in the mRS 90-day disability outcome between the groups was not considered as statistically significant (P = 0.134).
The modified Barthel index score of 0 (dead) patients was also less in the intervention group (7 vs. 15). The number of participants with severe, moderate, and mild dependency does not significantly differ between the groups (P = 0.239) [Table 2].
| Variables | Intervention (n=52) n(%) | Control (n=52) n(%) | P-value* |
|---|---|---|---|
| Disability (modified Rankin scale) | |||
| No dysfunction (0–1) | 12 (23.1) | 12 (23.1) | 0.134 |
| Dysfunction (2–5) | 33 (63.4) | 25 (48.1) | |
| Dead (6) | 7 (13.5) | 15 (28.8) | |
| Functional dependency (modified ADL Barthel index) | |||
| Mild or no dependence (>15) | 21 (40.4) | 20 (38.5) | 0.239 |
| Moderate dependence (10–15) | 13 (25.0) | 10 (19.2) | |
| Severe dependence (<10) |
11 (21.1) | 7 (13.5) | |
| Dead (0) | 7 (13.5) | 15 (28.8) | |
n: Frequency, %: Percentage, *Chi-square test, P≤0.05, ADL: Activities of daily living.
Kaplan–Meier survival curve between the intervention and control group shows a small divergence from the initial and expands over time till the 90-day follow-up. At 40 days, patients will survive 50% in the intervention group and 37% in the control group, according to the estimates [Figure 2]. The mean survival time for the intervention group was 33.03 (standard error [SE]-2.30) days, which was higher as compared to 30.69 (SE-5.09) days for the control group. The log-rank test shows a significant difference between the two survival curves in terms of the distribution from time to event (P = 0.019).
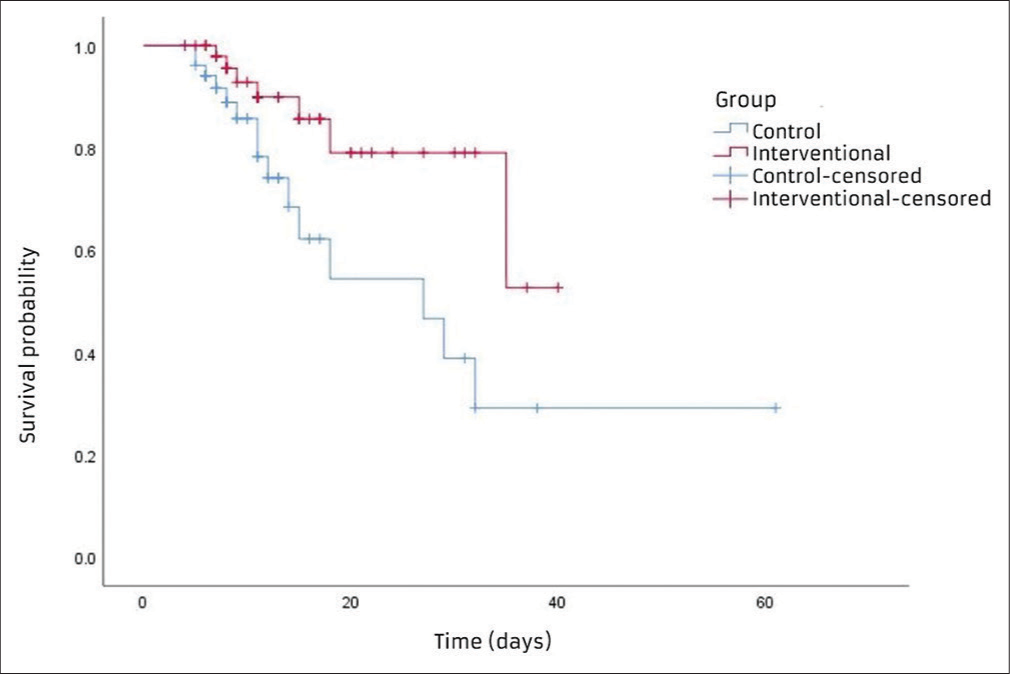
- Kaplan–Meier survival curves for both groups.
The all-cause mortality was also significantly higher in the control group at 28.8% compared to 13% in the intervention group with P < 0.05. The risk of death among control group participants is 2.143 times higher as compared to the participants in the FeSS intervention group. There is a 95% probability that the true risk ratio would be likely to lie in the range of 0.953–4.820 [Table 3].
| Group | Survival status | Risk ratio (95%CI) | Mean survival days (SE) | 95% CI | P-value* | |
|---|---|---|---|---|---|---|
| Alive n(%) | Dead n(%) | |||||
| Intervention | 45 (86.5) | 7 (13.5) | 2.14 (0.95–4.82) | 33.03 (2.30) | 28.51–37.54 | 0.019 |
| Control | 37 (71.2) | 15 (28.8) | 30.69 (5.09) | 20.72–40.67 | ||
n: Frequency, %: Percentage, CI: Confidence interval, SE: Standard error, *Log rank (mantel cox) test, P≤0.05
DISCUSSION
Hyperthermia, either due to infection or central fever, is commonly reported in acute stroke, with their prevalence being reported to be as high as 30% and 15%, respectively. These are associated with higher morbidity and mortality.[13-15] Literature that supports the unfavorable effect of hyperglycemia and dysphagia for stroke patients is also accumulating.[16,17] Targeted intervention in the acute phase of stroke to prevent complications and reverse these abnormal physiological changes is required to be implemented on a large scale.
Composite measures and strategies are known to reduce disability in acute stroke patients. The present study focuses on the measures that can be managed by nurses to enable implementation of an effective stroke care bundle in Indian hospitals. In addition to capturing the FeSS components (fever, glucose, and swallowing screening) and 90-day mortality, the present study uses outcome measures such as the mRS and modified ADL Barthel index to the effectiveness of the FeSS intervention, so as not to risk useful treatment effects of FeSS intervention.[18,19]
The FeSS intervention was found to reduce disability by 14–16% in a large-scale pre- and post-intervention study conducted in Australia.[20] However, the number of participants with no dysfunction remains the same in both groups in the present study. The number of participants with dysfunction (mRS 2–5) is higher in the intervention group (63.4%) than in the control group (48.1%). However, consideration should be given to the fact that the number of participants, who have died is comparably lower in the intervention group (13.5%) when compared to the control group (28.8%).
In several related studies, the outcome of MRS has been divided into two: No disability (mRS 0–1) and disability including death (mRS ≥2). Irrespective of stroke severity, the death and dependency (mRS ≥2) rates were significantly lower (Adjusted odds ratio 0.63, 95% confidence interval [CI] 0.41–0.97) among the participants receiving FeSS at 90 days follow-up.[12,21] On combining the death and dependency (mRS ≥2) for the present study, there is no difference in the number of participants with death and dependency between the groups. The functional dependency outcome measured by the modified ADL Barthel index also showed no significant difference in the number of participants across different categories of dependency between the groups.
A study by Middleton et al. on FeSS intervention reported a significantly lower mean temperature and mean glucose level within the first 72 h among patients, who received the FeSS intervention.[12] The present study does not find any significant difference in the mean temperature between the groups within the first 72 h of monitoring. Moreover, the mean glucose level in the intervention group was slightly higher than the control group (138.1 vs. 135.1). The number for insulin requirement (39 vs. 12 times) and paracetamol (10 vs. 7) was also higher in the intervention group, which can be attributed to the intensive FeSS monitoring. Febrile events were also significantly higher in the FeSS group (16.54% vs. 10.71%; P < 0.001) in a study assessing the effectiveness of FeSS intervention with electronic health records.[21]
While comparing all-cause mortality between the groups at 90 days, the FeSS group has significantly lower mortality with a 16% difference from the control group. A previous study on the effect of FeSS intervention on mortality reported no significant reduction in 90-day mortality alone (P = 0.36) despite a significant reduction in disability.[9] Adjusted analysis from another study also found that patients receiving FeSS were less likely to be dead or dependent after 90 days as compared to patients from the control group (P = 0•002).[12] The present study also reported the risk of mortality to be 2.143 (0.953–4.820) times higher in the control group. Implementing the FeSS protocol can be considered a protective factor against death among acute stroke patients (risk ratio > 0.822). Cox proportional hazard regression analysis from a related study showed that participants receiving FeSS intervention have improved survival of >20% (Adjusted hazard ratio: 0.77; 95% CI: 0.59–0.99; P = 0.045).[9] The Kaplan–Meier survival analysis in the present study also showed a significant difference of 15.3% in survival between the intervention and control groups.
The proportion of hemorrhagic stroke patients is higher in the present study as compared to other related studies, and since studies show that hemorrhagic stroke has poor outcomes compared to ischemic stroke, the outcome of mortality may be influenced by this.[3] Exploratory subgroup analysis from a previous FeSS trial reported that factors such as older age and increasing stroke severity were associated with poor survival (P < 0.001).[9] However, these factors were not explored in the present study. A larger multi-center study with a larger sample size can be conducted as an extension of the present study. A longer duration of participant followup can be done for a better assessment of the long-term effect of FeSS intervention. The introduction of other vital elements into the stroke care bundle can also be considered based on evidence. Assessment of other outcome measures apart from disability and mortality is also recommended for future studies.
CONCLUSION
Despite no significant reduction in 90-day disability and functional dependence, a significant reduction in mortality was noted among participants receiving the FeSS bundle of care. The FeSS interventions are simple and easy to apply with a good composite outcome and are suitable for developing countries. Therefore, the study suggested the promotion of nurse-led intervention using FeSS in the stroke units ensuring proper adherence for quality assurance.
Acknowledgment
We thank the nurse volunteers, who played a pivotal role in carrying out the FeSS intervention and for the completion of the study.
Ethical approval
The research/study is approved by the Jawaharlal Institute for Postgraduate Medical Education and Research (JIP/CON/ IEC/M.Sc/2020/MSN/I).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
JIPMER intramural research fund.
References
- Global, regional, and national burden of stroke and its risk factors 1990-2019: A systematic analysis for the global burden of disease Study 2019. Lancet Neurol. 2021;20:795-820.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of Stroke in India During 1960 to 2018: A systematic review and meta-analysis of community-based surveys. Neurol India. 2021;69:547-59.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrovascular disease In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
- [Google Scholar]
- Stroke in the 21st century: A snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018:3238165.
- [Google Scholar]
- Family-led rehabilitation after stroke in India: The ATTEND trial, study protocol for a randomized controlled trial. Trials. 2016;17:13.
- [CrossRef] [PubMed] [Google Scholar]
- Inclusion of a care bundle for fever, hyperglycemia, and swallow management in a National Audit for acute stroke: evidence of upscale and spread. Implement Sci. 2019;14:87.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus statement-suggested recommendations for acute stroke management during the COVID-19 pandemic: Expert Group on Behalf of the Indian Stroke Association. Ann Indian Acad Neurol. 2020;23:S15-23.
- [CrossRef] [PubMed] [Google Scholar]
- National Institutes of Health Stroke Scale: An alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke. 2020;51:282-90.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality reduction for fever, hyperglycemia, and swallowing nurse-initiated stroke intervention: QASC trial (Quality in Acute Stroke Care) follow-up. Stroke. 2017;48:1331-6.
- [CrossRef] [PubMed] [Google Scholar]
- The Barthel ADL index: A standard measure of physical disability? Int Disabil Stud. 1988;10:64-7.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a questionnaire to assess barriers to physical activity after stroke: The barriers to physical activity after stroke scale. Arch Phys Med Rehabil. 2019;100:1672-9.
- [CrossRef] [PubMed] [Google Scholar]
- Implementation of evidence-based treatment protocols to manage fever, hyperglycemia, and swallowing dysfunction in acute stroke (QASC): A cluster randomized controlled trial. Lancet. 2011;378:1699-706.
- [CrossRef] [PubMed] [Google Scholar]
- Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011;11:110.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemic stroke outcome: A review of the influence of post-stroke complications within the different scenarios of stroke care. Eur J Intern Med. 2016;29:9-21.
- [CrossRef] [PubMed] [Google Scholar]
- Fever, hyperglycemia, and swallowing dysfunction management in acute stroke: A cluster randomized controlled trial of knowledge transfer. Implement Sci. 2009;4:16.
- [CrossRef] [PubMed] [Google Scholar]
- Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci Ther. 2022;28:372-81.
- [CrossRef] [PubMed] [Google Scholar]
- Post-stroke dysphagia: Frequency, risk factors, and topographic representation: Hospital-based study. Egypt J Neurol Psychiatry Neurosurg. 2021;57:23.
- [CrossRef] [Google Scholar]
- Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43:1171-8.
- [CrossRef] [PubMed] [Google Scholar]
- Contemporary outcome measures in acute stroke research. Stroke. 2012;43:1163-70.
- [CrossRef] [PubMed] [Google Scholar]
- From QASC to QASCIP: Successful Australian translational scale-up and spread of a proven intervention in acute stroke using a prospective pre-test/post-test study design. BMJ Open. 2016;6:e011568.
- [CrossRef] [PubMed] [Google Scholar]
- A nurse-led multicomponent intervention supported by advanced electronic health records to improve the acute management of stroke patients: A pre-and post-intervention study. Int J Nurs Stud Adv. 2021;3:100023.
- [CrossRef] [Google Scholar]







