Translate this page into:
Effect of Pregabalin on Postcraniotomy Pain in Patients Undergoing Supratentorial Tumor Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial
Charu Mahajan, MD, DM Department of Neuroanaesthesiology and Critical Care, Neurosciences Centre, All India Institute of Medical Sciences New Delhi 110 029 India charushrikul@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background and Objectives Suboptimal management of postcraniotomy pain causes sympathetic and hemodynamic perturbations, leading to deleterious effects on the neurological system and overall patient outcome. Opioids are the mainstay of postoperative pain management but have various problems when given in high doses, or for prolonged durations in neurosurgical patients. The ideal method of pain control following craniotomy generally relies on a combination of various drugs. Oral pregabalin may be an attractive alternative in these patients.
Materials and Methods Sixty, American Society of Anesthesiologists class I and II patients posted for elective supratentorial craniotomy, aged 18 and 60 years, were randomly assigned into three groups of 20 each to receive oral placebo (Group A), pregabalin 75 mg (Group B), or pregabalin 150 mg (Group C) before the induction of anesthesia. At the end of the surgery, patient-controlled analgesia was started with intravenous fentanyl. Visual analog scale (VAS) score was recorded every 2 hours for 24 hours, along with total postoperative fentanyl requirement.
Results There were no differences in sex, duration of surgery or anesthesia and total intraoperative fentanyl administered among the three groups. The median postoperative VAS score (Group A—18.0, Group B—20, and Group C—22.0; p = 0.63) was similar in all the groups. However, postoperative fentanyl requirement over 24 hours was least in the group that received 150 mg pregabalin (Group A—190 μg, Group B—240 μg, and Group C—100 μg; p = 0.03).
Conclusions Even though pain scores were not significantly different, patients receiving 150 mg oral pregabalin required the least amount of postoperative opioids.
Keywords
analgesia
craniotomy
fentanyl
postoperative pain
pregabalin
visual analog scale
Introduction
The paradigm that craniotomy is associated with minimal postoperative pain is an age-old belief that has often led to inadequate analgesia in postcraniotomy patients. Pain can elicit a complex set of neural, cardiac, and respiratory changes that can lead to increased cerebral blood flow and intracranial pressure, cerebral edema, and even intracranial bleeding. Many patients may suffer from debilitating chronic pain syndromes.
Pregabalin is a structural analog of gamma-aminobutyric acid with analgesic, anticonvulsant, and anxiolytic effects.1 It is approved for treating neuropathic pain and used in diabetic peripheral neuropathy, postherpetic neuralgia, and central or peripheral neuropathic pain syndromes. Even though pregabalin has been evaluated for postoperative analgesia in spine surgery,2 laparoscopic hysterectomy,3 laparoscopic cholecystectomy,4 orthopaedic surgery,5 thoracotomy,6 and day-care gynecological laparoscopic surgery,7 there is a paucity of trials investigating its effects in attenuating postoperative pain after intracranial surgeries. This study was designed to evaluate the effect of preoperative pregabalin on postoperative pain and analgesic requirement in patients undergoing supratentorial craniotomy.
Materials and Methods
This randomized, double-blind, placebo-controlled study was conducted after approval from the institute’s ethics committee. Written informed consent was obtained from all the patients. Sixty patients posted for elective supratentorial craniotomy for tumor resection, between the ages of 18 and 65 years, American Society of Anesthesiologists physical status I and II and with Glasgow Coma Scale of 15, were included in the study. Patients with hepatic or renal diseases, mental disability, neurological deficits (precluding their use of a patient-controlled analgesia [PCA] device), large tumors (>50 mm in any dimension), and known allergy to the study medication or history of substance abuse were excluded from the study. A thorough general examination was performed 1 day prior to the surgery, and all significant details were noted. Patients meeting the inclusion criteria during the preanesthetic evaluation were explained about the use of PCA device and the 100-mm visual analog scale (VAS) for pain (0 = no pain; 100 = worst imaginable pain). Eligible patients were then randomly assigned to three groups of twenty each, with the help of a computer-generated table of random numbers, to receive oral placebo (Group A), pre-gabalin 75 mg (Group B), or pregabalin 150 mg (Group C). The patient and the observer were blinded to the three study groups. The drug containing envelope was prepared by a person who was not involved in this study. Each patient was given one envelope by a staff nurse who was unaware of the study. The patient was asked to take the capsule with a sip of water 1 hour before the induction of anesthesia.
Standard monitors included electrocardiography, pulse oximetry (SpO2), noninvasive blood pressure (NIBP), and capnography. General anesthesia was induced with propofol 1 to 2 mg/kg and fentanyl 2 μg/kg to achieve loss of response to verbal commands. Muscle relaxation was achieved with vecuronium 0.1 mg/kg. After tracheal intubation, anesthesia was maintained with nitrous oxide in oxygen (60:40) and isoflurane with a combined minimal alveolar concentration of 1.0 to 1.2. Fentanyl and vecuronium were supplemented as per the discretion of the consultant anesthesiologist. Radial artery cannulation was performed in all the patients for invasive blood pressure monitoring. Total intraoperative fentanyl used, duration of surgery and anesthesia were recorded. At the end of the surgery, residual neuromuscular blockade was reversed with neostigmine and glycopyrrolate. After tracheal extubation, all patients were shifted to the neurosurgical intensive care unit (NSICU). Assessment of pain, NIBP, heart rate, SpO2 and respiratory rate were done on arrival in the NSICU. Following this, patients were given a bolus of fentanyl 1 μg/kg through the PCA pump. The incremental dose was set at 0.25 to 0.5 μg/kg with a lock-out interval of 10 minutes and 4-hour limit of 0.4 mg. No background infusion of fentanyl was given. No other supplemental analgesic drugs or local anesthetics were given. VAS scores (100 mm) were recorded every 2 hours for the next 24 hours. A single observer who was blinded to the groups recorded all the measurements including severity of postoperative pain and postoperative fentanyl requirement. Side effects, if any, such as sedation, respiratory depression, pruritus, and nausea/vomiting, were recorded. In the event of any major problem (uncontrolled pain, sedation, obtundation, and severe respiratory depression), the PCA device was discontinued.
A sample size of 60 was chosen as a convenient number for this study based on feasibility issues of conducting this study at our institute. All statistical tests were performed using IBM SPSS Statistics, version 21 for Windows (IBM Corporation, New York, United States). Categorical variables such as sex were compared between the groups using the Pearson’s chi-squared test. Continuous variables were first assessed for normality of distribution. Baseline intraoperative continuous variables were then appropriately compared among the three groups using the Kruskal– Wallis test. The outcome variables including 24-hour VAS scores and 24-hour postoperative fentanyl requirement were expressed as median and range (interquartile range) and compared using the Kruskal–Wallis one-way analysis of variance. A p-value < 0.05 was considered statistically significant.
Results
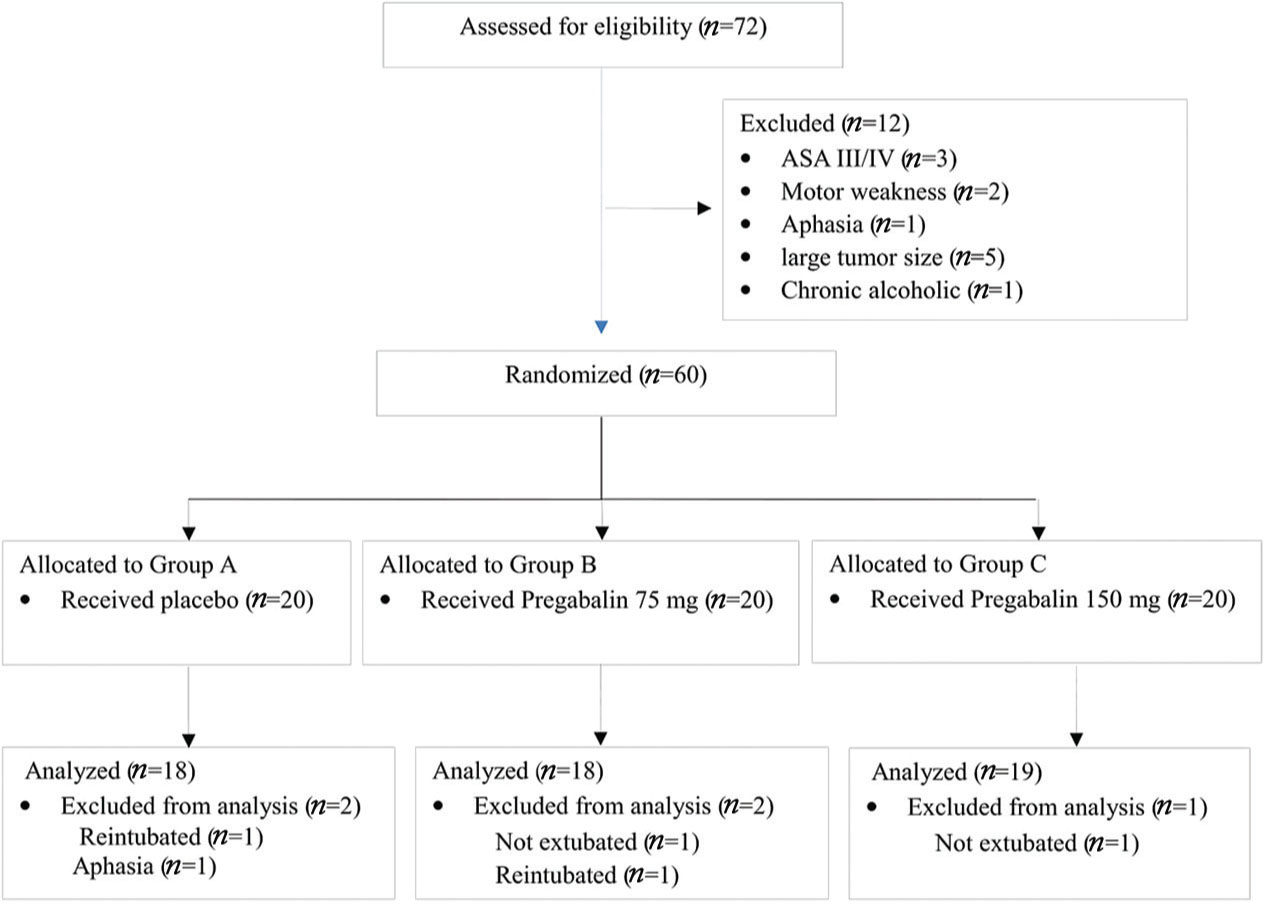
A total of 72 patients were assessed for eligibility (Fig. 1). Among these, 12 patients were excluded for not meeting the inclusion criteria, leaving 60 patients for randomization into three groups. Five patients were either not extubated or reintubated before 24 hours. These included two patients each in Group A and Group B and one patient in Group C. Scoring of pain and the use of PCA pump were not possible in these patients and they were excluded from the final analysis.
-
Fig. 1 CONSORT diagram. ASA III/IV, American Society of Anesthesiologists Class III/IV.
Fig. 1 CONSORT diagram. ASA III/IV, American Society of Anesthesiologists Class III/IV.
The median age of the patients in Group C was slightly lower compared with that of Groups A and B (p = 0.01) (Table 1). Males and females were evenly distributed (p = 0.77) in the three groups. There was no statistically significant difference in the duration of surgery (p = 0.72), duration of anesthesia (p = 0.96), or total intraoperative fentanyl administered (p = 0.21) among the three groups of patients (Table 1). In Group A, one patient required reintubation for pneumocephalus and another patient developed aphasia. In Group B, one patient was electively ventilated in the postoperative period for tense brain and another patient required reintubation in the ICU for seizures. In Group C, one patient was electively ventilated postoperatively for tense brain. These five patients were excluded from the final analysis. None of the patients experienced any side effects due to the study drug.
|
Variable |
Group A (n = 18) |
Group B (n = 18) |
Group C (n = 19) |
p-Value |
|---|---|---|---|---|
|
Note: Data presented as median, interquartile range (range), or na . Group A: placebo, Group B: pregabalin 75 mg, Group C: pregabalin 150 mg. |
||||
|
Age (y) |
35, 20 (18-65) |
49, 19 (20-60) |
30, 11 (19-57) |
0.01 |
|
Weight (kg) |
61, 15 (35-90) |
60, 7 (43-81) |
62, 17 (45-80) |
0.38 |
|
Sex (male/female) |
14/6 |
15/5 |
16/4 |
0.77 |
|
Surgery duration (min) |
248, 143.8 (105-420) |
240, 75 (140-560) |
245, 101 (90-600) |
0.72 |
|
Anesthesia duration (min) |
342, 191.3 (210-540) |
317.5, 77.5 (180-620) |
300, 145 (150-720) |
0.96 |
|
Intraoperative fentanyl (pg) |
275.0, 187.5 (100-475) |
300.0, 100.0 (225-500) |
300.0, 150.0 (100-1000) |
0.21 |
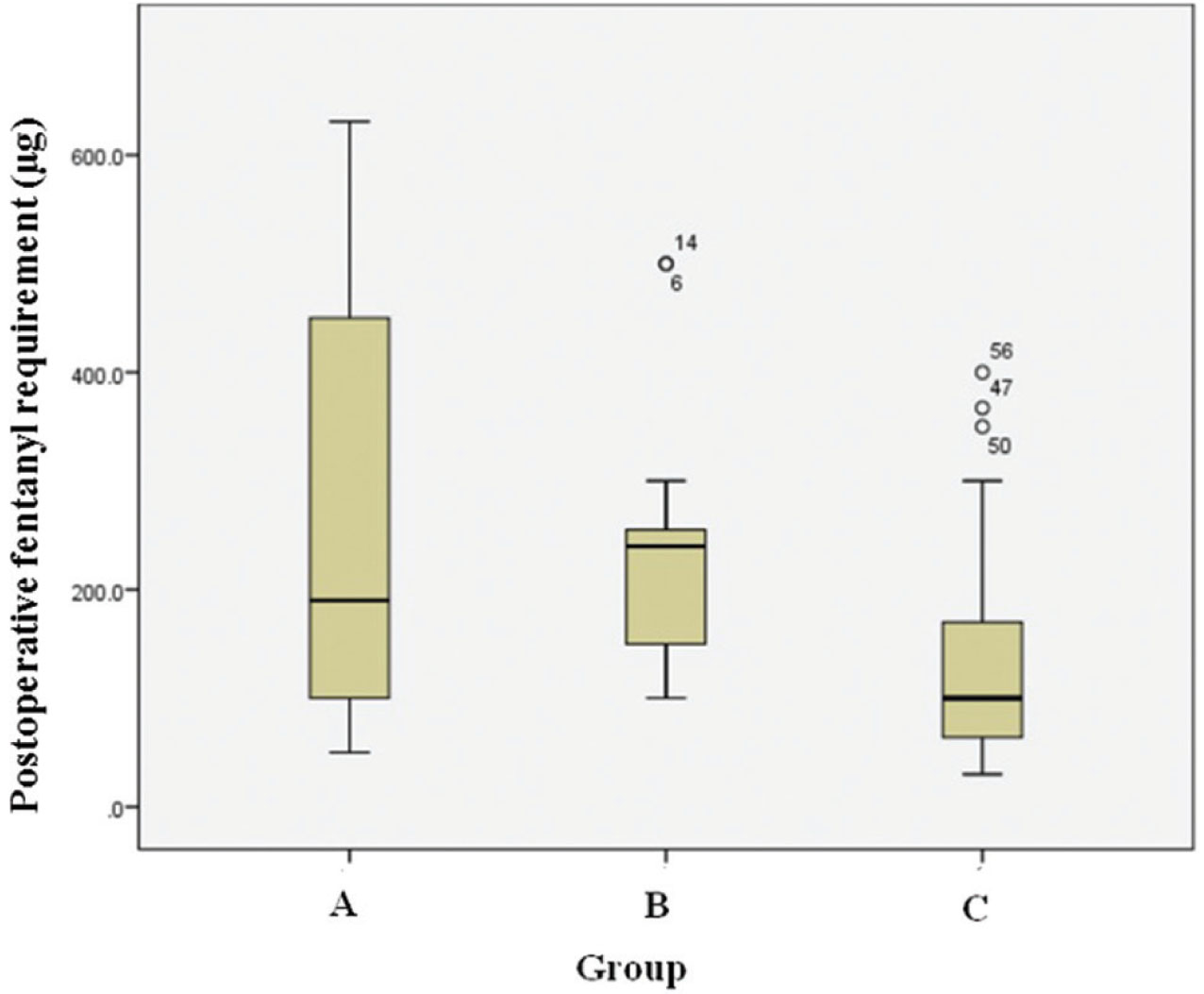
The median (interquartile range) VAS scores over 24 hours were 18 (16) in Group A, 20 (15) in Group B, and 22 (16) in Group C (Table 2). This difference was not statistically significant (p = 0.63). The requirement of postoperative fentanyl over 24 hours was least in Group C: 100.0 (130) μg, compared with 190.0 (365.0) μg in Group A and 240.0 (112.5) μg in Group B (Fig. 2). This difference was statistically significant (p = 0.03) (Table 2).
-
Fig. 2 24-Hour postoperative fentanyl consumption.
Fig. 2 24-Hour postoperative fentanyl consumption.
|
24-h details |
Group A (n = 18) |
Group B (n = 18) |
Group C (n = 19) |
p-Value |
|---|---|---|---|---|
|
Abbreviation: VAS, 100-mm visual analog scale. |
||||
|
Note: Data were presented as median and range (interquartile range); Group A: placebo, Group B: pregabalin 75 mg, Group C: pregabalin 150 mg. |
||||
|
VAS score |
18, 16 (10-40) |
20, 15 (22-33) |
22, 16 (25-35) |
0.63 |
|
Postoperative fentanyl (μg) |
190.0, 365.0 (50-1539) |
240.0, 112.5 (100-500) |
100.0,130.0 (30-400) |
0.03 |
Discussion
The severity of craniotomy pain can be significant, and more importantly, many patients may not get satisfactory pain relief because adequate analgesia is not prescribed. An old British survey more than 20 years back found that, in contrary to the widespread belief, moderate-to-severe pain is common after craniotomy and the pain responds very poorly to codeine.8 Yet, as recent as 2009, over two-third of the physicians in the United Kingdom still use codeine or dihydrocodeine as the first-line analgesic following craniotomy, using more potent opioids such as morphine, very sparingly.9 Opioids have been associated with undesirable side effects such as postoperative nausea and vomiting,10 respiratory depression,11 excessive sedation,12 neuropsychiatric manifestations,13 dizziness,14 and visual disturbances.14 Furthermore, there are concerns regarding its effects on cerebral hemodynamics and intracranial pressure.15 16 This has led to the widespread reservation about opioid use in neurosurgical patients, encouraging investigators to explore the effects of novel agents such as ketamine, gabapentinoids, and dexmedetomidine.
Gabapentinoids have been studied for postoperative pain relief after surgeries under general as well as regional anesthesia.17 There are several studies on general surgeries that have demonstrated that even a single preoperative dose of gabapentinoids can reduce postoperative pain. Agarwal et al4 found that preoperative oral pregabalin 150 mg was effective and safe for reducing both postoperative pain and fentanyl requirement in patients undergoing laparoscopic cholecystectomy. On the other hand, even though there was no improvement in VAS scores with pregabalin use in our study, there was a significant reduction in postoperative fentanyl requirement in those receiving 150 mg pregabalin. It is possible that patients undergoing neurosurgery behave differently than general surgical patients and that prolonged duration of neurosurgical procedures can reduce the analgesic effect of a single low dose (75 mg) of preoperative pregabalin (elimination half-life: 5.5–6.5 hours).
A meta-analysis also found that analgesic effect of pregabalin varied with the type of surgery—most apparent with short-duration gynecologic, laparoscopic, and ENT surgeries, with no clear benefit in some other types of surgeries.18 It is also established that preoperative oral pregabalin when given in doses up to 150 mg is safe and does not cause increase in postoperative sedation.12 Mishriky et al performed a meta-analysis in which they found that pregabalin not only improves postoperative analgesia but also causes increased sedation, dizziness, and visual disturbances.19 The authors studied the effect at different doses and regimens and found that the risk of sedation increased when pregabalin 300 mg was used as either single or multiple doses. However, we found no side effects associated with doses used in our study.
Studies of pregabalin in neurosurgical patients are scarce. There is only one published randomized study of pregabalin use for analgesia in patients undergoing craniotomy by Shimony et al.20 This study found that pregabalin attenuated pain scores and reduced analgesic consumption in postcraniotomy patients. Even though our findings are similar, their study design was very different as patients were given two doses of oral pregabalin (150 mg) before surgery, which they continued to receive postoperatively twice daily for 3 days. There may be some evidence to suggest that prolonged dosing protocols of pregabalin have better analgesic effects, but it is difficult to justify such protracted regimens in neurosurgical patients, where potential side effects such as undue sedation, dizziness, vomiting, and visual disturbances can mask the ongoing neurologic problems. Such extended pregabalin regimens for analgesia are unlikely to be adopted by most NSICUs. Our pregabalin dosing protocol using a single dose of 150 mg is extremely convenient to administer, is cost-effective, and is devoid of any major intraoperative or immediate postoperative side effects. Importantly, it reduces the requirement of postoperative opioids, mitigating some of the major concerns of using opioids in higher doses in neurosurgical patients.
It was beyond our scope to study the long-term effects of pregabalin. The limited sample size of the study did not permit data stratification based on the duration of the surgery, location and invasiveness of the tumor, the size of the craniotomy wound, or the degree of tissue dissection, all of which may have confounded the data. Larger trials, especially those employing different dose and duration protocols, and evaluating long-term outcome, are warranted to shed more light on the topic. The findings of this study can pave the way for designing such trials in neurosurgical patients in future.
Conclusions
Single oral dose of pregabalin 75 mg has no effect in lowering 24-hour mean VAS score or postoperative fentanyl requirement in patients undergoing supratentorial craniotomy. However, the findings of this study support the use of 150 mg pregabalin for preemptive analgesia before routine supratentorial tumor surgeries to reduce the requirement of postoperative opioids, unless specific contraindications for the use of the drug exist.
Conflict of Interest
None declared.
Funding The study was financially supported by the All India Institute of Medical Sciences, New Delhi, India.
References
- Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45(6):13-18.
- [Google Scholar]
- Perioperative pregabalin for postoperative pain control and quality of life after major spinal surgery. J Neurosurg Anesthesiol. 2012;24(2):121-126.
- [Google Scholar]
- A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008;134(1)Pain. 2008;134(2):106-112.
- [Google Scholar]
- Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth. 2008;101(5):700-704.
- [Google Scholar]
- Pregabalin and pain after total knee arthroplasty: a double-blind, randomized, placebo-controlled, multidose trial. Br J Anaesth. 2015;115(2):285-293.
- [Google Scholar]
- Effect of postoperative administration of pregabalin for post-thoracotomy pain: a randomized study. J Cardiothorac Vasc Anesth. 2015;29(6):1567-1572.
- [Google Scholar]
- Premedication with pregabalin 75 or 150 mg with ibuprofen to control pain after day-case gynaecological laparoscopic surgery. Br J Anaesth. 2008;100(6):834-840.
- [Google Scholar]
- A survey of post-craniotomy analgesia in British neurosurgical centres: time for perceptions and prescribing to change? Br J Neurosurg. 2009;23(5):538-542.
- [Google Scholar]
- Incidence and risk factors of postoperative nausea and vomiting in patients with fentanyl-based intravenous patient-controlled analgesia and single antiemetic prophylaxis. Yonsei Med J. 2014;55(5):1430-1435.
- [Google Scholar]
- In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two opioids have the same profile. Br J Pharmacol. 2015;172(2):532-548.
- [Google Scholar]
- The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg. 2009;108(4):1140-1145.
- [Google Scholar]
- Neuropsychiatric side effects due to a transdermal fentanyl patch: hallucinations. Am J Emerg Med. 2015;33(3):477.e1-477.e2.
- [Google Scholar]
- Effects of sufentanil on cerebral hemodynamics and intracranial pressure in patients with brain injury. Anesthesiology. 1995;83(4):721-726.
- [Google Scholar]
- The effect of alfentanil on cerebrospinal fluid pressure in human volunteers. Eur J Anaesthesiol. 1997;14(2):211-214.
- [Google Scholar]
- Preemptive gabapentin vs pregabalin for acute postoperative pain after surgery under spinal anaesthesia. Indian J Anaesth. 2008;52:829-834.
- [Google Scholar]
- Efficacy of pregabalin in acute postoperative pain under different surgical categories: a meta-analysis. Medicine (Baltimore). 2015;94(46):e1944.
- [Google Scholar]
- Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2015;114(1):10-31.
- [Google Scholar]
- Perioperative pregabalin for reducing pain, analgesic consumption, and anxiety and enhancing sleep quality in elective neurosurgical patients: a prospective, randomized, double-blind, and controlled clinical study. J Neurosurg. 2016;125(6):1513-1522.
- [Google Scholar]






