Translate this page into:
Double-barrel Y-configuration Stenting for Flow Diversion of a Giant Recurrent Basilar Apex Aneurysm with the Pipeline Flex Embolization Device
Address for correspondence: Dr. Dale Ding, Department of Neurosurgery, University of Virginia, P. O. Box: 800212, Charlottesville, VA 22908, USA. E-mail: dmd7q@hscmail.mcc.virginia.edurecurred
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Giant basilar apex aneurysms are extremely challenging to successfully manage. The Pipeline Flex embolization device (PFED) is a new generation flow-diverting stent with a modified delivery system which allows resheathing of the stent after partial deployment. We describe a case of double-barrel Y-configuration stenting of a giant, recurrent basilar apex aneurysm using the PFED. A 73-year-old male was previously treated for an unruptured 11-mm basilar apex aneurysm with stent-assisted coiling using a Neuroform stent. The aneurysm was retreated twice with repeat coiling. After the third recurrence and persistent aneurysm growth into a giant, symptomatic lesion, we decided to proceed with flow diversion. We performed Y-stenting of the basilar bifurcation using three PFEDs, and was recoiled the aneurysm sac. Due to the low porosity of the flow diverters, a side-by-side double-barrel configuration was necessary in the basilar artery. Without the PFED's resheathable capability, it would not have been possible to perform Y-stenting with flow diverters.
Keywords
Basilar artery
endovascular procedures
intracranial aneurysm
Pipeline Flex embolization device
Y-stenting
INTRODUCTION
Large basilar apex aneurysms harbor high risks of hemorrhage and represent some of the most difficult cerebrovascular lesions to successfully treat.[12] Due to the excessive morbidity associated with microsurgical clipping of large basilar apex aneurysms, endovascular treatment has become the mainstay of treatment at most institutions.[3] The Pipeline Flex embolization device (PFED; Medtronic, Minneapolis, Minnesota, USA) is a new generation flow-diverting stent, in which the delivery system of the pipeline embolization device (PED) was modified to allow resheathing of a partially deployed stent.[4] The aim of this case report is to describe our technique for the treatment of a giant, recurrent basilar apex aneurysm with double-barrel, Y-configuration stenting using the PFED.
CASE REPORT
A 73-year-old male was incidentally diagnosed with a large, 11-mm basilar apex aneurysm with a posteriorly projecting dome [Figure 1a and b]. The aneurysm was initially treated with stent-assisted coil embolization, during which a 4.5 mm × 30 mm Neuroform EZ stent (Stryker Neurovascular, Fremont, California, USA) was deployed from the right posterior cerebral artery (PCA) into the basilar artery [Figure 1c and d]. The aneurysm subsequently recurred and was retreated with coiling 20 months later. The aneurysm recurred second time 15 months after the first retreatment, and the recurrence was coiled again. Follow-up neuroimaging 10 months after the second retreatment showed recanalization and significant growth of the aneurysm sac, which was causing new-onset edema in the left thalamus. Clinically, the patient developed memory deficits and occipital headaches.
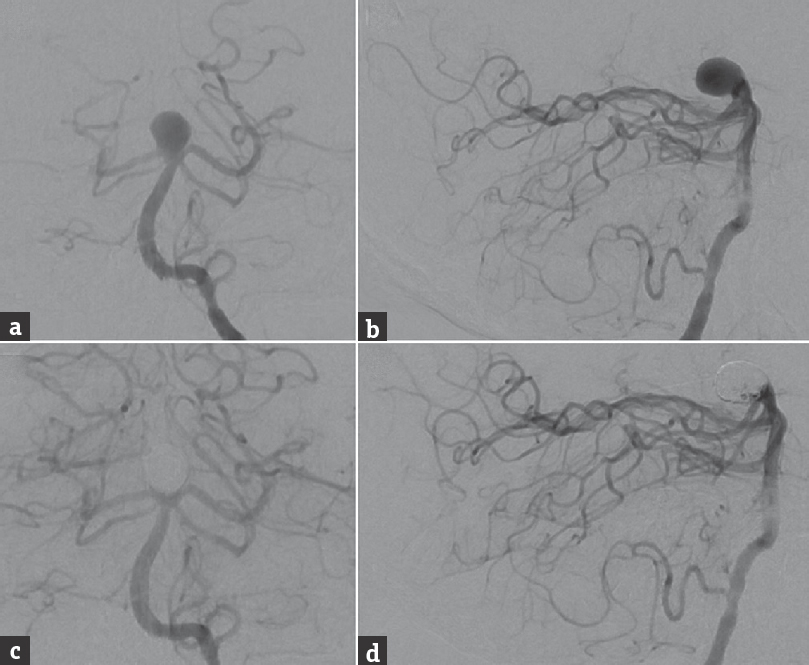
- Preoperative cerebral angiography, (a) anterior-posterior and (b) lateral views of a left vertebral artery injection, shows a large, 11-mm wide-necked basilar bifurcation aneurysm with a posteriorly oriented dome. Postoperative angiography after endovascular treatment with stent-assisted coil embolization using a 4.5 mm × 30 mm Neuroform EZ stent deployed from the right posterior cerebral artery to the basilar artery, (c) anterior-posterior, and (d) lateral views of a left vertebral artery injection, shows a small neck remnant
Given giant size of the recurrent basilar apex aneurysm despite multiple endovascular procedures and the patient's neurological deterioration, we elected to treat the aneurysm with flow diversion using a double-barrel, Y-configuration PFED construct [Figure 2a and b]. After general endotracheal anesthesia was induced, femoral artery access was obtained bilaterally. A 5-French (Fr) ENVOY catheter (Codman Neuro, Raynham, Massachusetts, USA) was navigated, through a 6-Fr right femoral sheath, to the right vertebral artery (VA). A Navien 072 distal access catheter (DAC; Medtronic) was navigated, through a 6-Fr Cook Shuttle sheath (Cook Medical, Bloomington, Indiana, USA), to the left intradural VA. A Marksman microcatheter (Medtronic) was navigated, through the ENVOY catheter in the right VA, to the right PCA P3 segment, and a second Marksman microcatheter was navigated, through the DAC, to the left PCA P2 segment. An Echelon 10 microcatheter (Medtronic) was navigated into the recurrent aneurysm sac through the DAC.
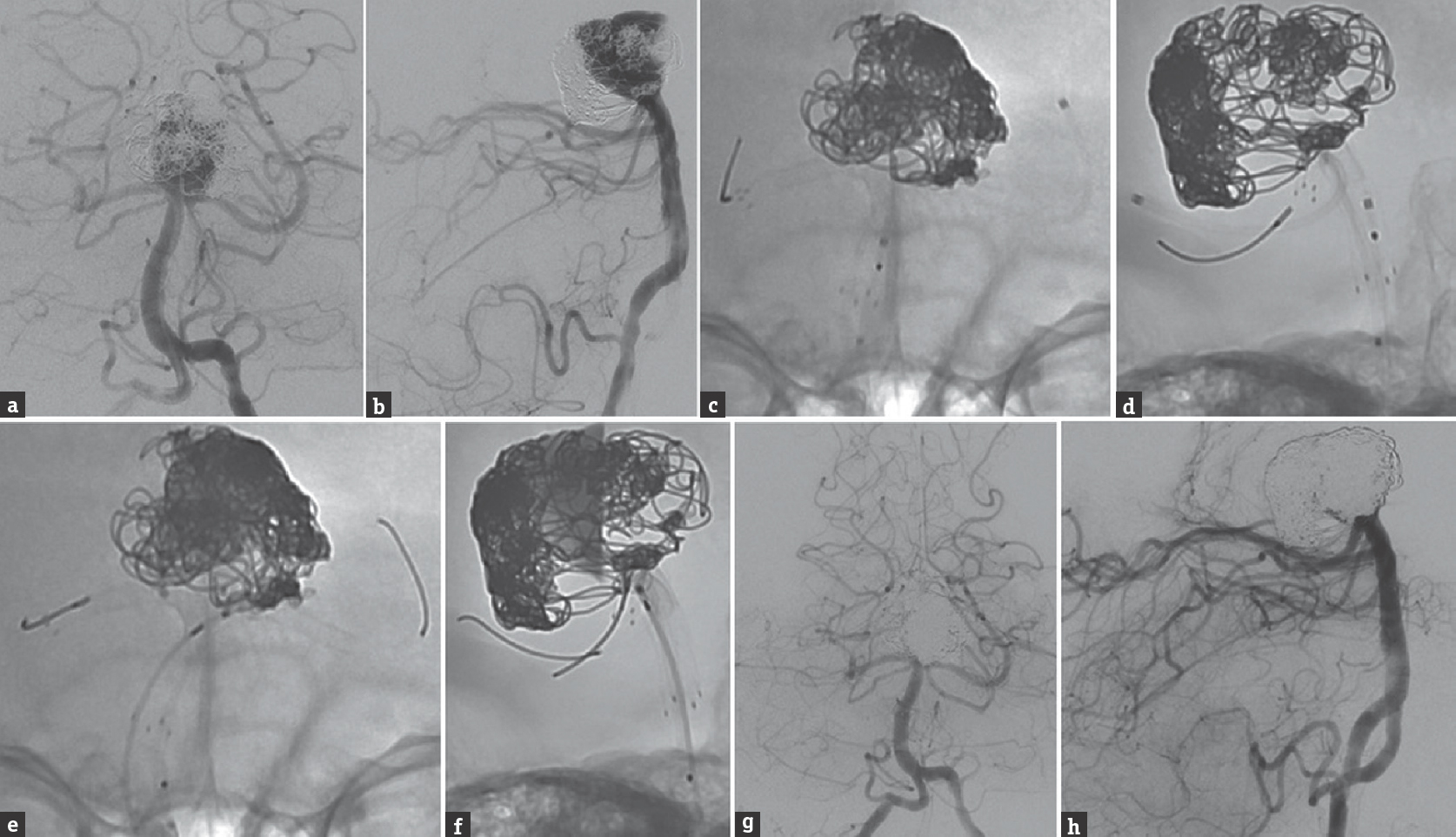
- Cerebral angiography, (a) anterior-posterior and (b) lateral views of a left vertebral artery injection, shows significant recurrence of the previously stent-coiled and twice recoiled basilar apex aneurysm. Unsubtracted angiography, (c) anterior-posterior and (b) lateral views of a right vertebral artery injection, shows the first PFED deployed through the in situ Neuroform stent; (e) anterior-posterior and (f) lateral views of a left vertebral artery injection, shows partial deployment of the second PFED from the left posterior cerebral artery back to the level of the Neuroform stent in the basilar artery. Control angiography after the residual aneurysm sac was coiled, the second PFED was fully deployed, and a third PFED was telescoped within the initial PFED from the right posterior cerebral artery to the basilar artery, (g) anterior-posterior and (h) lateral views of a right vertebral artery injection, shows a small amount of residual aneurysm filling. PFED: Pipeline Flex embolization device
First, a 2.75 mm × 14 mm PFED was deployed within the existing Neuroform stent, from the right PCA to the basilar artery at the level of the superior cerebellar arteries, and held in position with its delivery catheter [Figure 2c and d]. A second 2.5 mm × 14 mm PFED was partially deployed from the left PCA back to the level of the Neuroform stent, alongside the first PFED [Figure 2e and f]. A mixture of bare platinum and hydrocoils was deployed into the recurrent aneurysm through the Echelon 10 microcatheter. The second PFED in the left PCA was fully deployed, and held in position with the delivery system. The base of the aneurysm was then coiled further to achieve a greater packing density (total of 32 coils). A third 2.75 mm × 12 mm PFED was telescoped within the first PFED and deployed from the right PCA P1 segment into the basilar artery, forming a double-barrel configuration with the second left-sided PFED. The third PFED was used to extend the flow diversion construct proximal to the previously deployed Neuroform stent. The final control angiography showed patency of both PCAs, with a small degree of residual aneurysm filling [Figure 2g and h].
The patient had an uncomplicated postoperative course and was discharged home on dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg daily). Follow-up magnetic resonance angiography performed 10 months after the flow diversion procedure showed persistent filling of the aneurysm as well as interval occlusion of the left PCA [Figure 3]. Unfortunately, despite multiple attempts, we have not been able to obtain a follow-up catheter angiogram on the patient.
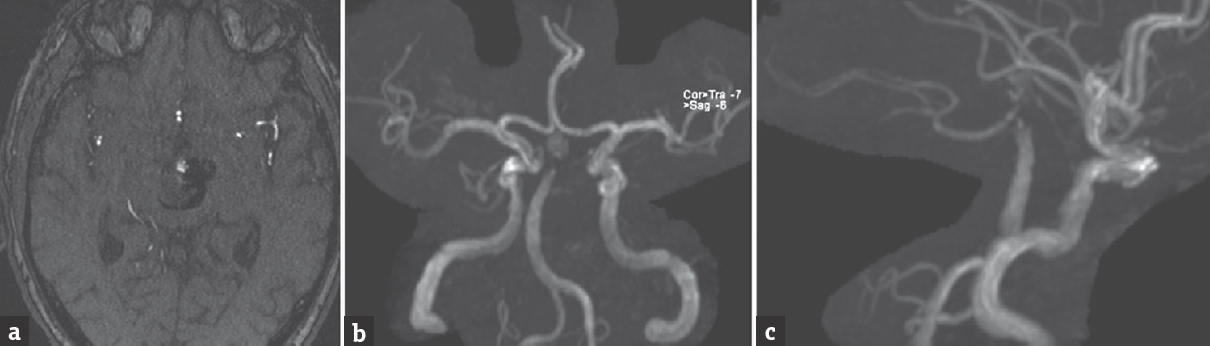
- Follow-up magnetic resonance angiography 10 months after the flow diversion procedure, (a) axial time-of-flight and three-dimensional reconstruction, (b) anterior-posterior and (c) lateral views, shows residual aneurysm filling and interval occlusion of the left posterior cerebral artery
DISCUSSION
The advent of flow-diverting stents, such as the PED, has drastically altered the landscape of endovascular aneurysm treatment.[567] However, one of the disadvantages of the first generation PED was its inability to be resheathed after the initiation of deployment, which was associated with a risk of complications, such as suboptimal stent position, stent malapposition to the vessel wall, endoleak, thromboembolism, and parent artery occlusion.[8910] The PFED is the same flow-diverting stent as the PED but uses a different delivery system, which allows resheathing after deployment of over 90% of the length of the stent.[4] This improvement not only allows facilitating more precise stent deployment but also enables treatment of more complex aneurysms.
In our case of a giant, recurrent basilar apex aneurysm which had previously been treated with stent-assisted coiling and two additional sessions of repeat coiling, we believed that flow diversion was the best option to promote complete aneurysm obliteration. However, the morphological features of the basilar bifurcation and the presence of the prior Neuroform stent limited our options for flow-diverting stent placement. We decided to exploit the resheathability of the PFED to perform Y-configuration flow diversion of the basilar bifurcation. Y-stenting is typically implemented with higher porosity stents, during which one stent is deployed within another.[11] However, the low porosity of flow diverters requires a side-by-side (i.e., double-barrel) configuration when performing Y-stenting. This treatment strategy would not have been technically feasible with the first generation PED.
Kono and Terada evaluated the hemodynamic characteristics of several different stenting configurations for bifurcation aneurysms using an in vitro flow model, and found that the “kissing-Y” and “crossing-Y” configurations resulted in the greatest reductions of flow velocity within an aneurysm.[12] Our double-barrel Y-stent construct would have been classified by the aforementioned article as a “kissing-Y” configuration. At the time, we performed the first retreatment for recurrence, we did not believe that the use of another conventional self-expanding stent (e.g., a Neuroform stent), deployed into the left PCA in a “crossing-Y” configuration, would have had a favorable risk to benefit profile. However, this may be a reasonable treatment option for recurrent bifurcation aneurysms although its safety and efficacy compared to flow diversion is not well defined.
CONCLUSIONS
The PFED's ability to be resheathed before deployment allows Y-configuration stenting of complex bifurcation aneurysms. However, these bifurcation aneurysms are associated with a number of critical perforator arteries, the occlusion of which can lead to significant neurological deficits. Therefore, careful consideration must be given to alternative approaches. For appropriately selected patients with particularly challenging basilar apex aneurysms, double-barrel Y-stenting with the PFED can be an effective treatment technique. Further studies are necessary to determine the safety profile of flow diversion across perforator-rich arterial segments.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-10.
- [Google Scholar]
- Potential role of aspirin in the prevention of aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis. 2015;39:332-42.
- [Google Scholar]
- Perforator aneurysms of the posterior circulation: Case series and review of the literature. J Neurointerv Surg. 2013;5:546-51.
- [Google Scholar]
- Preliminary experience with the Pipeline Flex Embolization Device: Technical note. J Neurointerv Surg. 2015;7:748-51.
- [Google Scholar]
- Endovascular treatment of recurrent intracranial aneurysms following previous microsurgical clipping with the Pipeline Embolization Device. J Clin Neurosci. 2014;21:1241-4.
- [Google Scholar]
- Endovascular treatment of ophthalmic artery aneurysms: Ophthalmic artery patency following flow diversion versus coil embolization. J Neurointerv Surg. 2016;8:919-22.
- [Google Scholar]
- Cavernous carotid aneurysms: A new treatment paradigm in the era of flow diversion. Expert Rev Neurother 2016 Epub ahead of print
- [Google Scholar]
- Microsurgical extraction of a malfunctioned Pipeline Embolization Device following complete deployment. J Cerebrovasc Endovasc Neurosurg. 2013;15:241-5.
- [Google Scholar]
- DynaCT imaging for intraprocedural evaluation of flow-diverting stent apposition during endovascular treatment of intracranial aneurysms. J Clin Neurosci. 2014;21:1981-3.
- [Google Scholar]
- Microsurgical strategies following failed endovascular treatment with the Pipeline Embolization Device: Case of a giant posterior cerebral artery aneurysm. J Cerebrovasc Endovasc Neurosurg. 2014;16:26-31.
- [Google Scholar]
- Technology developments in endovascular treatment of intracranial aneurysms. J Neurointerv Surg. 2016;8:135-44.
- [Google Scholar]
- Hemodynamics of 8 different configurations of stenting for bifurcation aneurysms. AJNR Am J Neuroradiol. 2013;34:1980-6.
- [Google Scholar]






