Translate this page into:
Diabetes Mellitus as the Presenting Feature of Friedreich's Ataxia
Address for correspondence: Dr. Meenal Garg, Department of Pediatric Neurosciences, EEG Room, 2nd Floor, Bai Jerbai Wadia Hospital for Children, Parel, Mumbai - 400 012, Maharashtra, India. E-mail: docmeenal@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Patients with Friedreich's ataxia (FA) are at an increased risk of developing diabetes mellitus and glucose intolerance. Diabetes usually develops many years after the initial presentation. We report an 8-year-old girl who initially presented with diabetic ketoacidosis and was treated as a case of insulin-dependent diabetes mellitus. Around a year later, she developed gait problems and ataxia. Cardiac involvement was detected on echocardiography. Genetic testing confirmed the diagnosis of FA. FA should be a diagnostic consideration in children presenting with diabetes and neurological issues, even with early presentation of the former. Early occurrence of diabetes and rapid progression of ataxia in this patient needs a better understanding of underlying genetic mechanisms.
Keywords
Cardiomyopathy
diabetes
diabetes mellitus
Friedreich's ataxia
sensory neuropathy
INTRODUCTION
Friedreich's ataxia (FA) was first described in 1863 by Nikolaus Friedreich as a “degenerative atrophy of the posterior columns of the spinal cord, leading to progressive ataxia, sensory loss, and muscle weakness, often associated with scoliosis, foot deformity, and heart disease.” It is an autosomal recessive inherited disease caused by a homozygous expansion of a guanine-adenine-adenine (GAA) trinucleotide repeat in intron 1 of the frataxin gene on chromosome 9 in most patients.[1] Patients with FA are at an increased risk of developing diabetes mellitus and glucose intolerance, with variable incidence reported in literature. However, diabetes has rarely been reported as the presenting feature of FA. Here, we report an 8-year-old girl who was found to have diabetes mellitus Type-1 and was later diagnosed with FA.
CASE REPORT
An 8-year-old girl with insulin-dependent diabetes mellitus was referred to the Pediatric Neurology Department for gait problems. She had been asymptomatic till 7 years of age when she developed polyuria, polydipsia, and acute onset vomiting along with high blood sugars, increased urine and blood ketones, and metabolic acidosis. A diagnosis of diabetic ketoacidosis with underlying diabetes mellitus was made, and she was started on insulin along with dietary and lifestyle modifications. The child responded well and was on regular follow-up with well-controlled sugars and normalization of hemoglobin A1c levels. Around a year later, she complained of gait problems. She had developed gradual onset progressive imbalance for 3 months, had frequent falls, and could not climb stairs without support. She could use her upper limbs well and did not have problems with handwriting. There was a history of slipping of slippers but no sensory loss. Examination revealed an ataxic gait with an inability to tandem walk or stand on one leg. There was no vision, hearing, speech, or cognitive issues. Deep tendon reflexes were absent in the lower limbs and power was 4/5 in distal lower limb muscles and normal elsewhere. Plantar reflexes were normal. She had loss of vibration sense in the lower limbs, but other sensations were preserved.
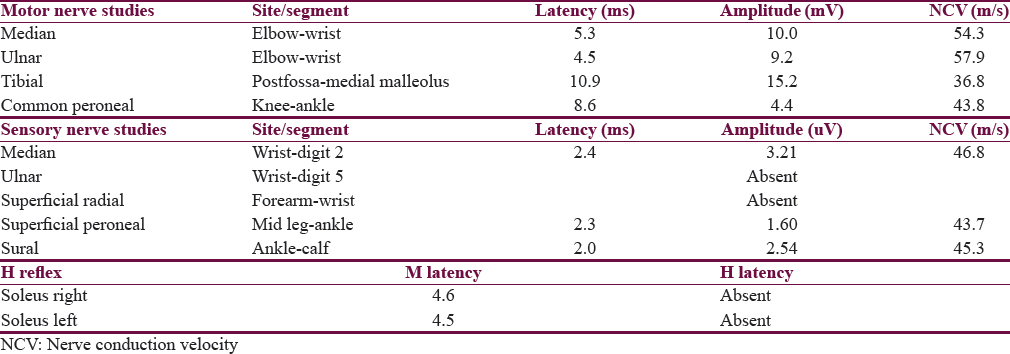
Nerve conduction velocity studies revealed an axonal type of generalized sensory neuropathy [Table 1]. The presence of diabetes mellitus along with early-onset sensory neuropathy raised the suspicion of FA, and a two-dimensional echocardiogram was ordered. There was left ventricular (LV) concentric hypertrophy with infiltration (LV mass – 76.1 g) with impairment of LV function (ejection fraction – 52.5%). Trinucleotide repeat study for FA showed abnormally expanded GAA repeats on both alleles (>200), thus confirming the diagnosis. The patient was started on idebenone and enalapril along with continued management for diabetes. Ophthalmological and orthopedic screening examinations were normal. Parents were found to be heterozygous carriers for the mutation. She has been started on physical and occupational therapy and is on regular follow-up. Genetic counseling has been provided to the family.
DISCUSSION
FA is one of the most common inherited ataxias, reported largely in Caucasian populations.[2] The disease affects central and peripheral nervous systems, heart, skeleton, and endocrine pancreas. It is characterized by progressive gait and limb ataxia, dysarthria, lower limb areflexia, decreased vibration sense, muscular weakness of the legs, and extensor plantar response.[23] Hypertrophic cardiomyopathy and increased incidence of diabetes mellitus are well known. Clinical diagnostic criteria have been developed by Geoffroy et al.[4] and Harding,[3] but genetic testing is confirmatory. The gene, mapped to chromosome 9q13-221.1, codes for a mitochondrial protein called frataxin. Most common mutation is GAA-repeat expansion, with FA patients having 70–1700 repeats, most commonly in the 600–900 range. Up to 4% of patients are heterozygotes, harboring a GAA expansion on one chromosome and a point mutation or small deletion on the other.[4]
The exact function of frataxin is unclear, but it has been demonstrated that its deficiency leads to a disruption of iron−sulfur enzymes, mitochondrial iron overload, and increased sensitivity to oxidative stress.[5] Genotype-phenotype correlations in FA have been the subject of research as there is considerable heterogeneity, especially with regard to the age of onset, progression, and the presence of cardiomyopathy and diabetes. The relation of size of trinucleotide expansion with clinical features is established, with higher size of repeats being associated with a more severe phenotype. A higher incidence of diabetes has been found in some studies[6] and none in others.[7] A recent study has found that nondiabetic FA patients with longer GAA repeats on the shorter allele showed higher incidence of glucose intolerance.[8]
Diabetes in FA has been presumed to occur due to defects in insulin action or decreased insulin secretion from β islet cells in the pancreas. However, it is likely that both these mechanisms contribute to the pathogenesis, with β cell dysfunction being a precondition for glucose intolerance.[9] The molecular pathways are likely to be similar to those in neurological disease, with in vitro and in vivo models pointing toward mitochondrial dysfunction, mitochondrial apoptosis, and endoplasmic reticulum stress.[1011]
The reported incidence of diabetes in FA varies from 6% to 19%, with nearly 49% patients found to have impaired glucose tolerance in a recent study.[3479] Some differences might be due to change in the diagnostic criteria for diabetes, diagnostic tests, and availability of genetic diagnosis.[12] The average time of occurrence of diabetes is around 10–15 years after the diagnosis.[3] Our patient is unusual in that she presented only with diabetes and did not report any neurological features at presentation. Subtle neurological involvement was possibly present earlier, but overt symptoms appeared only later. A few patients with FA who presented with diabetes have also been reported recently.[1314] However, these patients were older, had considerable neurological features at presentation, and were previously being investigated for them. In addition, our patient has shown a rapid progression of disease in the period of 1 year with appearance of ataxia, neuropathy, and cardiac involvement. The impact of diabetes on the course of disease and severity of neuropathy in this patients remains to be seen.
CONCLUSION
This is an unusual report of diabetes as the initial presentation in a patient with FA. This patient has also shown accelerated course of disease. However, the genetic mechanisms accounting for phenotypic variations in FA remain to be fully elucidated. In patients with diabetes who present with early sensory neuropathy or ataxia, FA should be a consideration even in the pediatric age group. A better understanding of molecular mechanisms will certainly pave the way for improved therapeutic strategies in the near future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Friedreich's ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423-7.
- [Google Scholar]
- Friedreich's ataxia: A clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981;104:589-620.
- [Google Scholar]
- Clinical description and roentgenologic evaluation of patients with Friedreich's ataxia. Can J Neurol Sci. 1976;3:279-86.
- [Google Scholar]
- Understanding the molecular mechanisms of Friedreich's ataxia to develop therapeutic approaches. Hum Mol Genet. 2010;19:R103-10.
- [Google Scholar]
- The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am J Hum Genet. 1996;59:554-60.
- [Google Scholar]
- Friedreich's ataxia. Revision of the phenotype according to molecular genetics. Brain. 1997;120(Pt 12):2131-40.
- [Google Scholar]
- Effects of genetic severity on glucose homeostasis in Friedreich ataxia. Muscle Nerve. 2016;54:887-94.
- [Google Scholar]
- Central role and mechanisms of ß-cell dysfunction and death in Friedreich ataxia-associated diabetes. Ann Neurol. 2012;72:971-82.
- [Google Scholar]
- Death protein 5 and p53-upregulated modulator of apoptosis mediate the endoplasmic reticulum stress-mitochondrial dialog triggering lipotoxic rodent and human ß-cell apoptosis. Diabetes. 2012;61:2763-75.
- [Google Scholar]
- First presentation of diabetes as diabetic ketoacidosis in a case of Friedreich's ataxia. Clin Diabetes. 2015;33:84-6.
- [Google Scholar]
- A rare presentation of Friedreich's ataxia in pediatric case: Diabetes mellitus. J Clin Res Pediatr Endocrinol. 2015;7:72.
- [Google Scholar]






