Translate this page into:
Dengue-associated autoimmune encephalitis presenting as a dystonic storm in a young male: A rare case report
*Corresponding author: Dr. Biswamohan Mishra, Department of Neurology, All India Institute of Medical Sciences, New Delhi, India. biswamohan26@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sidharth S, Vibha D, Singh RK, Tripathi M, Elavarasi A, Gaikwad SB, et al. Dengue-associated autoimmune encephalitis presenting as a dystonic storm in a young male: A rare case report. J Neurosci Rural Pract. doi: 10.25259/JNRP_356_2024
Abstract
While dengue fever is known to cause various neurological manifestations, such as dengue encephalitis and post-infectious immune-mediated syndromes, Autoimmune Encephalitis (AIE) following dengue remains an under-recognized entity. We report a case of a young male who presented with refractory seizures and a dystonic storm following recovery from dengue fever. Despite normal magnetic resonance imaging findings, 18F-FDG PET imaging revealed hypermetabolism in the basal ganglia and hippocampus, suggestive of AIE. Notably, the autoimmune and paraneoplastic panel was negative. The patient demonstrated a marked clinical response to immunotherapy, underscoring the importance of early recognition and treatment. This case highlights the need for clinicians to consider AIE as part of the spectrum of post-dengue neurological complications, even in the absence of typical imaging findings, to facilitate timely diagnosis and intervention.
Keywords
Dengue
Dystonia
Dystonic disorders
Autoimmune diseases of the nervous system
Encephalitis
INTRODUCTION
Dengue fever is known to cause various neurological manifestations, including dengue encephalitis and post-infectious immune-mediated conditions such as acute inflammatory demyelinating polyneuropathy and acute disseminated encephalomyelitis (ADEM).[1] Dengue can affect the central nervous system (CNS) during the infection through active viral invasion or post-infectious autoimmune attack.[2] Autoimmune encephalitis (AIE) following dengue fever infection is a rare but known complication.[3] Here, we present a case of post-dengue AIE following recovery from dengue fever, characterized by refractory seizures and a dystonic storm. The absence of abnormalities on magnetic resonance imaging (MRI) but hypermetabolism on 18 fluorodeoxyglucose-positron emission tomography (18F-FDG PET) imaging involving the basal ganglia and hippocampus highlights the diagnostic challenges associated with this condition. This rare presentation underscores the importance of heightened clinical suspicion and vigilance in identifying post-dengue AIE, as timely recognition and initiation of immunotherapy can improve patient outcomes.
CASE REPORT
A 15-year-old male presented with a history of acute febrile illness with epistaxis six months back and was detected to have dengue fever (serum non-structural 1 antigen positive) and managed conservatively. Two weeks after the onset of this febrile illness, he developed altered sensorium, multiple episodes of seizures in the form of atonic seizures, unknown onset generalized tonic-clonic seizures, generalized myoclonic jerks along with stiffness of both upper and lower limbs and intermittent abnormal posturing involving trunk and limbs. Suspecting ADEM despite normal brain imaging, he was treated with pulse steroids with an injection of methylprednisolone 1 g for five days and intravenous immunoglobulin (Ig) (2 g/kg) with improvement in the seizure frequency. His sensorium also improved and he was conscious and followed occasional commands, smiled at family members, and maintained eye contact but was mute. However, the intermittent abnormal posturing of the limbs and trunk persisted. He was discharged on anti-seizure medications and without any maintenance immunotherapy. At discharge, he was modified Rankin score (mRS) of 5. Subsequently, he had a recurrence of seizures after four months, and increased posturing of the limbs and trunk for which he presented to us. Examination revealed a conscious but akinetic and mute male with generalized distal predominant myoclonic jerks, spasticity of bilateral upper and lower limbs, intermittent sustained posturing of the trunk and distal upper and lower extremities, without any loss of consciousness during these movements, quadriparesis, diminished Deep Tendon Reflexes and bilateral mute plantar response. Although the patient was occasionally responsive to commands, she was not sufficiently cooperative to allow completion of the rest of the neurological examination. Primary clinical considerations were seronegative AIE, post-dengue AIE, or fulminant presentation of neurodegenerative disorders such as progressive myoclonic epilepsy and subacute sclerosing pan encephalitis. Routine hematological, biochemical, and metabolic parameters, including ceruloplasmin, urinary copper, ferritin, lactate, and serum creatine kinase levels, revealed no abnormality [Supplementary Table S1]. Electroencephalogram (EEG) showed diffuse delta-theta slowing without any epileptiform discharges. There were no EEG changes also corresponding to the abnormal posturing movements, suggesting that these movements were likely dystonia. MRI brain and spine with contrast revealed no parenchymal abnormality but diffuse cerebral atrophy compared to his previous imaging [Figure 1a-f]. Cerebrospinal fluid (CSF) analysis revealed that nil cells, protein 66 mg/dL, sugar 74 mg/dL [Random blood sugar (RBS): 145 mg/dL], and cultures were negative. Serum and CSF autoimmune and paraneoplastic antibody panel [Supplementary Table S1] were negative. CSF pan viral polymerase chain reaction (PCR), including IgM of Japanese encephalitis and dengue, anti-measles antibody was negative. The serum dengue IgM was positive. F-18 FDG PET showed hypermetabolism in both basal ganglia and mesial temporal regions with occipital hypometabolism suggestive of AIE [Figure 1g-i]. He received methylprednisolone 1 g intravenous once daily (OD) for five days followed by five cycles of plasmapheresis (40 mL/kg/day each cycle) and two doses of rituximab 1 g two weeks apart for long-term immunosuppression. With this, there was an improvement in spasticity and dystonia and he became free of myoclonic jerks and seizures. However, he continued to be mute. He was discharged on oral prednisolone 40 mg OD, levetiracetam 500 mg twice a day, valproate 500 mg twice a day, clonazepam 0.25 mg thrice a day, lacosamide 100 mg twice a day, and levodopa/carbidopa 100/25 mg ½ tablet thrice a day. At three-month follow-up, the patient was mRS 4 with improvement in his power in upper and lower limbs to 3/5 and just able to walk with support. He was seizure-free, had no dystonia, and eye contact was maintained and continued to be mute. He is being planned for the maintenance dose of injection rituximab.
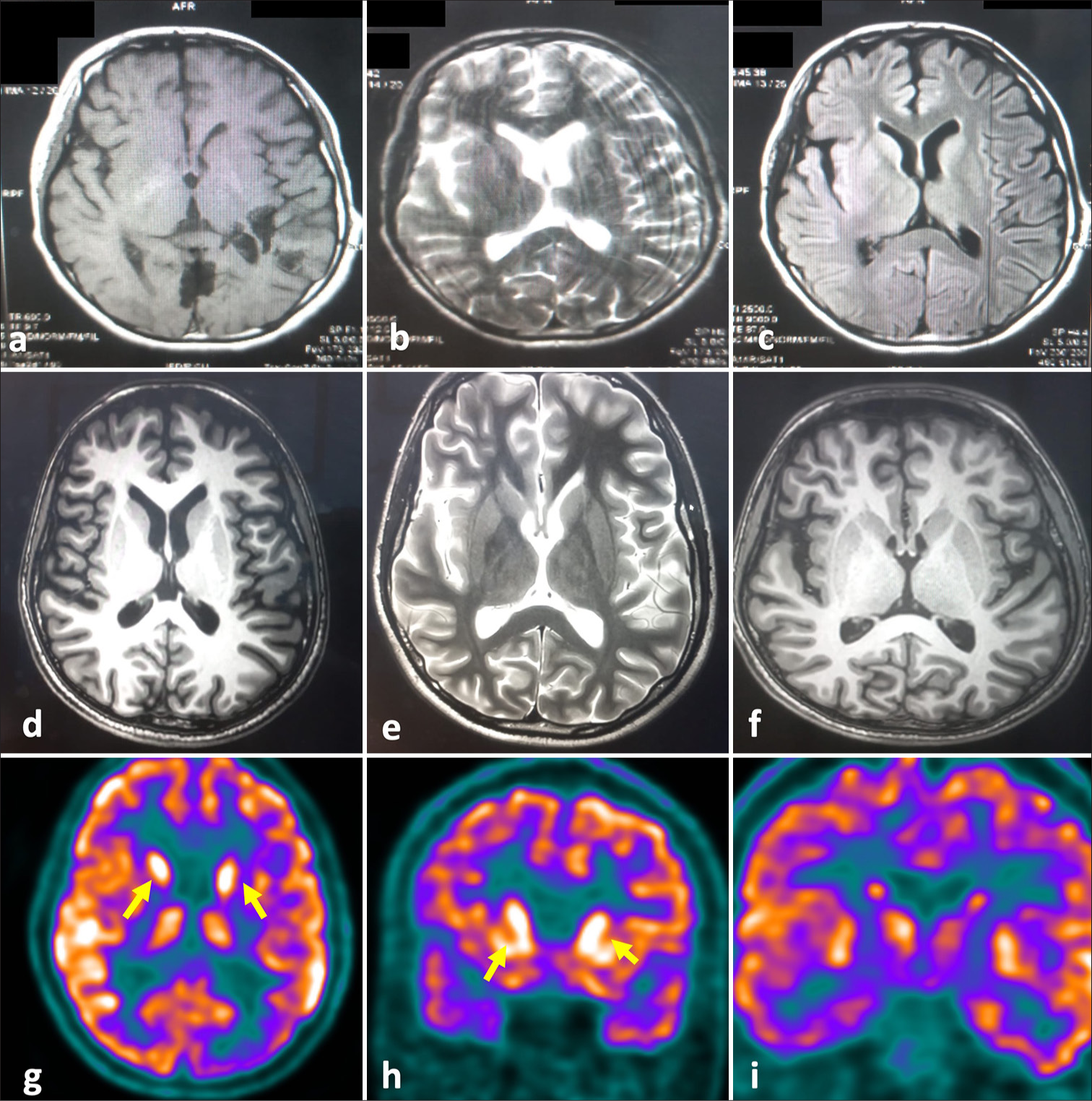
- The brain magnetic resonance imaging (MRI) images and 18FDG-PET images of the patient. (a-c) showing brain MRI images of the patient at the time of initial hospitalization six months before the presentation to us. (a) T1 axial, (b) T2 axial, and (c) T2 FLAIR axial images of brain show no abnormality. (d-T1 axial, e-T2-axial) showing brain MRI images at the time of admission depicting cerebral atrophy compared to the previous imaging. (f) depicts T1 post-contrast axial section of brain showing no evidence of contrast enhancement. (g-i) F-18 FDG PET shows hypermetabolism in both basal ganglia (yellow arrows) and mesial temporal regions with occipital hypometabolism suggestive of autoimmune encephalitis.
DISCUSSION
The present case report highlights a rare presentation of post-dengue AIE with dystonic storms and seizures. While post-dengue-associated ADEM is a known complication, this case emphasizes the need for clinicians to be aware of the broader spectrum of neurological manifestations that may follow dengue fever, including AIE. This case report also emphasizes the importance of clinical suspicion, particularly when imaging and other diagnostic tests are negative. A review of literature on dengue-related neurological manifestations showed a case series of 17 seropositive IgM dengue patients, who were evaluated for neurological manifestations, out of which 11 patients presented with encephalopathy of which three patients had seizures, one patient had myoclonus.[4] Out of 11 patients with encephalopathy, eight patients showed slowing on EEG, and one patient showed changes on globus pallidus on MRI brain.[4] In another retrospective cohort study, 63,814 laboratories confirmed that dengue cases in Taiwan were analyzed to study the spectrum of autoimmune disease after dengue infection as compared to control, which showed a high incidence rate of 27.73/10,000 person-year. Forty-two patients had developed autoimmune encephalomyelitis-like syndrome within 30 days of dengue infection.[5] In our case, the patient had slowing on EEG without any signal changes in the MRI brain. Solomon et al. screened 378 patients of CNS infections and identified 21 seropositive dengue patients, out of which seven were primary infections and 13 were secondary, and only three patients had dengue antibodies detected in CSF.[6] Ten patients had serum PCR positive for dengue. The most frequent manifestations were reduced consciousness and seizures.[6] Bhushan et al., in their cross-sectional observational study, noted 79 cases of immune-mediated neurological complication post-dengue with a subacute latency period of 7-30 days where conditions such as Guillain-Barré syndrome (GBS), brachial neuritis, polyneuritis cranialis, Miller fisher syndrome, and limbic encephalitis were seen.[3] A dengue-associated neurological attack can be seen as encephalitis in the acute phase or as a post-infectious immune-mediated attack causing limbic encephalitis as in this case report. Our case also depicts the presentation of dystonia as a clinical feature in the spectrum of this neurological disease which is believed to be present in more than 10% of cases of dengue.[7,8] IgM dengue may play a role in supplementing the diagnosis on a clinical background. FDG PET is a useful investigation that can support a clinical suspicion of AIE by demonstrating basal ganglia hypermetabolism and cortical hypometabolism which had majorly supplanted the clinical diagnosis in this case. Metabolic topography of AIE was demonstrated in 24 antibodies positive (anti-NMDA-15, anti-VGKC/LGI-1-6, and anti-GAD-3) with hypometabolism most commonly parietal and occipital cortex and hypermetabolism in basal ganglia supporting the diagnosis on FDG PET.[9] Further, research is needed to understand the underlying mechanisms of post-dengue AIE and to identify optimal diagnostic and treatment strategies for this rare condition.
CONCLUSION
This case underscores the importance of recognizing AIE as a potential post-dengue complication, particularly in patients with persistent neurological symptoms. It highlights the role of advanced imaging, in diagnosis when conventional imaging is inconclusive, and emphasizes the need for prompt immunotherapy to achieve favorable outcomes. Awareness of such rare manifestations is crucial for timely diagnosis and management.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Neurological complications of dengue fever. Curr Neurol Neurosci Rep. 2022;22:515-29.
- [CrossRef] [PubMed] [Google Scholar]
- Neurological manifestations of dengue infection. Front Cell Infect Microbiol. 2017;7:449.
- [CrossRef] [PubMed] [Google Scholar]
- Immune-mediated neurological manifestations of dengue virus-a study of clinico-investigational variability, predictors of neuraxial involvement, and outcome with the role of immunomodulation. Neurol India. 2018;66:1634-43.
- [CrossRef] [PubMed] [Google Scholar]
- Neurological manifestations of dengue virus infection. J Neurol Sci. 2006;244:117-22.
- [CrossRef] [PubMed] [Google Scholar]
- Re-examination of the risk of autoimmune diseases after dengue virus infection: A population-based cohort study. PLoS Negl Trop Dis. 2023;17:e0011127.
- [CrossRef] [PubMed] [Google Scholar]
- Neurological manifestations of dengue infection. Lancet Lond Engl. 2000;355:1053-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dystonic storm: A practical clinical and video review. J Clin Mov Disord. 2017;4:10.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of movement disorders associated with dengue encephalitis. MRIMS J Health Sci. 2022;10:109.
- [CrossRef] [Google Scholar]
- Metabolic topography of autoimmune nonparaneoplastic encephalitis. Neuroradiology. 2018;60:189-98.
- [CrossRef] [PubMed] [Google Scholar]







