Translate this page into:
Delayed-onset Reversible Cortical Blindness after Resuscitation from Cardiac Arrest
Address for correspondence: Dr. Aaron de Souza, Department of Neurology, Goa Medical College, Bambolim - 403 202, Goa, India. E-mail: adesouza1@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We present a patient who presented with cortical blindness (CB) 1 week after repeated cardiac arrest while undergoing treatment for an acute myocardial infarction. He had been revived within 5 min in each instance and was apparently neurologically normal until presentation. Magnetic resonance imaging showed subtle hyperintensities on fluid-attenuated inversion recovery and diffusion-weighted imaging in both temporooccipital cortices. A rapid recovery over the next 2 weeks was remarkable for the appearance of metamorphopsia. CB may present even days to weeks after hypoxic-ischemic encephalopathy following cardiac arrest, even in patients apparently without immediate neurological sequelae. The pathogenesis of this phenomenon remains to be fully elucidated, but is likely to be due to delayed effects of anoxia on the occipital cortex and may be analogous to the previously described syndrome of delayed posthypoxic leukoencephalopathy. Prognosis for visual recovery appears to be good.
Keywords
Cardiac arrest
cortical blindness
hypoxic-ischemic encephalopathy
occipital cortex
INTRODUCTION
Cortical blindness (CB) is defined as visual loss secondary to damage to the geniculocalcarine visual pathways,[1] and is characterized by complete loss or gross impairment of vision and loss of optokinetic nystagmus, with normal pupillary light reflex, ocular motility, retina, and fundi. Visual loss is often incomplete. Such blindness has been recognized to arise from a variety of pathological processes affecting the occipital lobes, most commonly hypoxia, ischemia, or meningoencephalitis.[2] Although a well-recognised complication of hypoxic-ischemic encephalopathy (HIE) following cardiac arrest, it is usually apparent immediately on regaining consciousness after resuscitation. We present an unusual patient who developed delayed CB 1 week after resuscitation from repeated cardiac arrests sustained after an acute myocardial infarction. The possible pathogenesis and relationship to the syndrome of delayed posthypoxic leukoencephalopathy (DHPL) are discussed.
CASE REPORT
A 47-year-old male presented to a local hospital with acute precordial chest pain in shock, and an electrocardiogram revealed an acute anteroseptal myocardial infarction. He developed a complete heart block, for which he was referred emergently to the regional cardiology center. En route, he sustained three episodes of cardiac arrest and was revived successfully from each within 3 min. A primary coronary angioplasty with stenting to the left main coronary artery was performed, with good recovery of the left ventricular function and resolution of the arrhythmia. No neurologic deficits were noted on the 2nd day of hospitalization. Inotropic support was withdrawn the next day, and he remained in the cardiology unit for a week on oral medication.
One week after presentation, he woke up with an acute visual loss in both eyes. Perception of light was preserved, but he was unable to appreciate hand movements. An ophthalmologic examination was normal, with normal pupillary reflex and optic fundi. He did not have any other neurologic deficits. Blood chemistry and a computed tomography (CT) scan of the brain were normal, and visual evoked potentials showed attenuated waveforms from either eye. Gradual recovery was noted from the 3rd day, with slow improvement in visual acuity. He complained of metamorphopsia: straight lines – for example, fluorescent tubes – appeared curved when viewed directly. By the 10th day, he was able to read, and visual acuity was recorded as 6/60 in both eyes. A magnetic resonance imaging (MRI) of the brain at this time showed subtle hyperintensity of the cerebral cortex in both temporooccipital regions on fluid-attenuated inversion recovery and diffusion-weighted imaging [Figure 1], and visual field charting was normal [Figure 2]. He continued to improve steadily, and 1 month afterward had only minor visual complaints with normal visual acuity, color vision, field of vision, and reading ability.
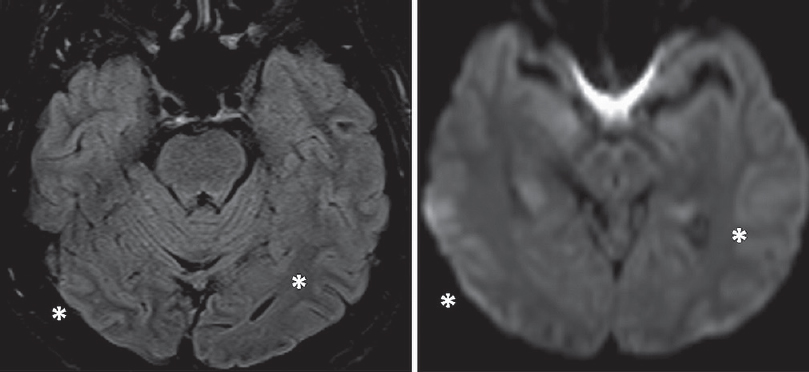
- Magnetic resonance imaging of the brain 10 days after onset of neurological symptoms shows subtle hyperintensities in bilateral parietooccipital cortices on fluid-attenuated inversion recovery and diffusion-weighted imaging (asterisks)
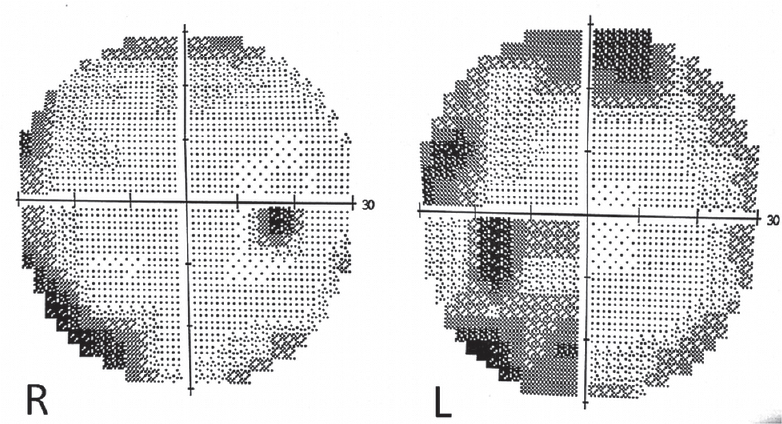
- Visual fields were normal 2 weeks after onset of blindness
DISCUSSION
Delayed posthypoxic CB is an extremely rare event in which visual loss following HIE becomes evident after an interval without neurological symptoms. The preservation of pupillary reflexes, presence of metamorphopsia and imaging findings serve to localize pathology in our patient to the occipital cortex. Hysteria is excluded by the absence of reflex blinking to menace.[2] We are aware of only one other brief report of this condition, which described CB in a patient 1 week after an episode of pulseless electrical activity lasting 5 min. Her MRI showed patchy diffusion restriction and T2 hyperintensity in both occipital lobes. As in our patient, substantial visual recovery occurred.[1]
Circulatory failure due to HIE or hypoxia after cardiac arrest deprives the cerebral cortex of essential nutrients and oxygen,[3] even leading to occipital lobe infarction. CB after cardiac arrest may result from transient cerebral hypotensive episodes causing more severe tissue hypoxia in the border zones among the major cerebral arteries (i.e., involving the primary visual, sensory, and motor cortices with their association areas).[4] The gray matter of the adult brain is particularly vulnerable to acute HIE, in which a complex cascade of cellular events resulting in glutamate excitotoxicity, release of free radicals, and apoptosis typically causes selective radiologic and pathologic insult to gray matter.[3]
The pathogenesis of delayed CB, occurring a few days after cardiac arrest without intervening neurological symptoms, is less certain. Based on extrapolations from similar cases, in which CB or other neurological symptoms occurred a few days after diverse brain insults, it may be linked to delayed and cumulative oxidative stress, delayed effects of anoxia, or cortical laminar necrosis affecting layers 3 and 4 of the striate cortex.[156] Other aspects of pathogenesis such as variability in delay of onset, involvement of isolated areas of the neuraxis in an individual, severity of symptoms, and underlying pathological variations still remain unexplained.
A parallel may be drawn with the unusual syndrome of DHPL, where patients manifest a neurological relapse after initial recovery or clinical stability from an acute hypoxic episode. Patients usually present 2–40 days (median, 23) after the initial event with relatively lucid intervening periods of variable lengths.[378] Most commonly noted after carbon monoxide intoxication – 2.75% of patients exposed to carbon monoxide presented in this way[7] – it is also observed following hypoxia of other causes, including respiratory failure in drug overdoses.[8] A proposed mechanism relates to the fact that the turnover rates for some myelin-related proteins range between 19 and 22 days, which is close to the average time for clinical relapse after the initial injury.[8] Outcome was variable, but complete recovery was documented in 75% of patients within 1 year.[3]
Our patient underwent a coronary angiogram as part of the management of his cardiac condition. Although transient CB is a recognized complication of cerebral angiography, only isolated case reports describe its occurrence after cardiac catheterization and none beyond a few hours after the procedure. CT scans demonstrate marked contrast enhancement of the occipital lobes, which was not seen in our case.[9]
In cases of CB due to nonvascular or nonsurgical causes, the prognosis is generally good, and substantial improvement can be expected, particularly in younger patients. Even after hypotensive episodes during cardiac surgery, 60% regained normal vision. Of children with CB, 25%–100% recovered useful vision, although the rate of recovery is variable and they may be left with a complex disorder of visual perception.[2] Recovery is due to normalization of the postlesionally dysfunctional cortex by resolution of edema or restitution of axonal connections, the use of minute parts of preserved field, or “nonstriate vision.”[4] Visual hallucinations and perceptual problems may become apparent during recovery, and are the clinical correlate of the electrophysiological hyperexcitability of the recovering partially damaged visual cortex.[10] Visual cognitive, field and visual perceptual deficits may persist for a long time.[4] There is no correlation between the duration of CB and the extent of visual recovery.
CONCLUSION
CB may present even days to weeks after HIE following cardiac arrest, even in patients apparently without immediate neurological sequelae. The pathogenesis of this phenomenon remains to be fully elucidated, but is likely to be due to delayed effects of anoxia on the occipital cortex and may be analogous to the previously described syndrome of DHPL. Prognosis for visual recovery appears to be good.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Reversible delayed posthypoxic leukoencephalopathy. AJNR Am J Neuroradiol. 2006;27:1763-5.
- [Google Scholar]
- Acute cerebral blindness in childhood. Six cases studied clinically and electrophysiologically. Neurology. 1970;20:1147-56.
- [Google Scholar]
- Late onset reversible cortical blindness following electrocution. Clin Neurol Neurosurg. 2015;139:311-3.
- [Google Scholar]
- Delayed postanoxic encephalopathy after strangulation. Serial neuroradiological and neurochemical studies. Arch Neurol. 1991;48:871-4.
- [Google Scholar]
- Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40:433-5.
- [Google Scholar]
- Delayed post-hypoxic leukoencephalopathy: Case report with a review of disease pathophysiology. Neurol Int. 2013;5:e13.
- [Google Scholar]
- Transient cortical blindness after cardiac catheterization with iobitridol. Tex Heart Inst J. 2007;34:373-5.
- [Google Scholar]
- Visual hallucinations in recovery from cortical blindness: Imaging correlates. Arch Neurol. 2000;57:561-5.
- [Google Scholar]






