Translate this page into:
Delayed Facial Palsy after Microvascular Decompression: Report of Two Cases
Address for correspondence: Dr. G. Lakshmi Prasad, Department of Neurosurgery, Room 12, OPD Block, Kasturba Hospital, Manipal - 576 104, Karnataka, India. E-mail: lakshmi.prasad@manipal.edu
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Microvascular decompression (MVD) is a novel surgical procedure predominantly performed for treating trigeminal neuralgia (TN) and hemifacial spasm (HS). Multiple studies have proven the long-term success of MVD for both these conditions. The most common complications of MVD reported include chemical meningitis, facial hypesthesia, cerebrospinal fluid leak, facial paresis, and hearing loss. Delayed facial palsy (DFP) is an uncommon complication mostly noted in MVD for HS and after the removal of acoustic tumors. We report two cases of DFP occurring after performing MVD, one each for HS and TN. This is also the first case of DFP to be reported after MVD for TN. Both were young females who developed DFP 2 weeks after surgery. They were managed with oral steroids and acyclovir for 2–3 weeks and achieved excellent outcome at an average of 4.5 weeks from the onset. We conclude that although majority of the cases improve spontaneously, steroids and acyclovir might assist in faster recovery.
Keywords
Delayed facial palsy
hemifacial spasm
herpes virus
microvascular decompression
steroids
trigeminal neuralgia
INTRODUCTION
Popularized by Peter Jannetta, microvascular decompression (MVD) has remained the gold standard surgical treatment for trigeminal neuralgia (TN) and hemifacial spasm (HS) and has provided consistent and excellent long-term results.[1234] Delayed facial palsy (DFP) is an uncommon complication after MVD mostly noted after surgery for HS.[345] DFP is often relatively sudden in onset and usually occurs over a 24-h period after surgery.[34] The incidence ranges from 2.8% to 8.3% for those undergoing MVD for HS, but till date, none has been reported after MVD for TN.[123456] We report two cases of DFP occurring in young females after undergoing MVD surgeries for TN and HS (one case each). This is the first case of DFP reported after MVD for TN. The possible etiopathogenesis, risk factors, time course of evolution, prognosis, and outcomes of this complication are discussed with a pertinent literature review.
CASE REPORTS
Case 1
A 39-year-old female with no comorbidities presented with severe right-sided facial pain that was sudden, sharp shooting type and noted along the V2, V3 distribution. This had troubled her for the last 5 years and had worsened for the last 6 months. The pain had initially responded to oxcarbazepine and gabapentin but was refractory for the last 6 months. Furthermore, the pain had changed in character from an intermittent one to a more continuous type, and she could barely talk for more than a minute and also had difficulty in eating because of her pain. Neurological examination was unremarkable. Magnetic resonance imaging (MRI) brain showed evidence of neurovascular conflict at the root entry zone of right trigeminal nerve by the superior cerebellar artery loop [Figure 1]. In addition, the right V nerve was atrophic in comparison with the left V nerve. There were no compressive lesions adjacent to the trigeminal nerve. A retrosigmoid approach was used. After opening the dura, cerebellum was gently retracted superomedially and cerebrospinal fluid (CSF) released. The fifth nerve was found thinned out, and a loop of SCA was noted to compress the nerve root entry zone. Under microscopic magnification, the vessel was separated from the trigeminal nerve and Teflon felt was placed between them. There was no handling of the facial nerve during surgery. Postoperatively, her trigeminal pain resolved immediately, but she developed mild (House–Brackmann [HB] Grade 2) facial paresis and ipsilateral sensorineural hearing loss, probably related to the cerebellar retraction during exposure. She was discharged on the postoperative day (POD) 4 on her previous medications. Two weeks after surgery, she returned with worsening of her facial weakness to HB Grade 4 palsy. She denied having any fever/vesicles around the ear in the recent past. Except for the presence of craniectomy defect, nothing was evident on computed tomography (CT) such as hematoma or infarct. With a diagnosis of DFP, she was prescribed oral steroids (dexamethasone 4 mg TID for 1 week and BD for 1 week) and acyclovir (400 mg TID for 2 weeks). After 4 weeks of DFP onset, her facial paresis had improved to her immediate postoperative status. She is presently on regular follow-up in our outpatient department relieved of pain but still on her previous medications.

- Magnetic resonance imaging brain (three-dimensional constructive interference in steady-state sequences) showing the loop of superior cerebellar artery (black arrow) abutting the root entry zone of the right trigeminal nerve (white arrow). Further, the right fifth nerve is atrophic as compared to the left side (arrowhead). No mass lesion is evident
Case 2
A 25-year-old female presented with right-sided involuntary facial twitching, predominantly over the eyelid and angle of mouth for the last 12 months. Neurological examination was unremarkable. MRI showed evidence of neurovascular conflict at the root entry zone of the facial nerve by the anterior inferior cerebellar artery (AICA) [Figure 2]. After a retrosigmoid approach, the cerebellum was gently retracted superomedially and cisternal CSF released. The AICA vessel loop was found to be compressing the seventh nerve. Using standard microsurgical methods, the vessel and nerve were separated and Teflon felt was placed between them. From POD-1, her facial spasms resolved completely, but she developed mild HB Grade 2 facial palsy and was discharged on POD-4. Ten days after surgery, she developed worsening of her facial palsy to HB Grade 4 severity. She had no history of fever, auricular vesicles/rash. Imaging was essentially normal, except for the craniotomy defect. She was given a course of oral prednisolone starting at 1 mg/kg/day for 1 week and tapered over the next 2 weeks. After a follow-up of 7 weeks (5 weeks after DFP), her facial palsy had completely resolved.
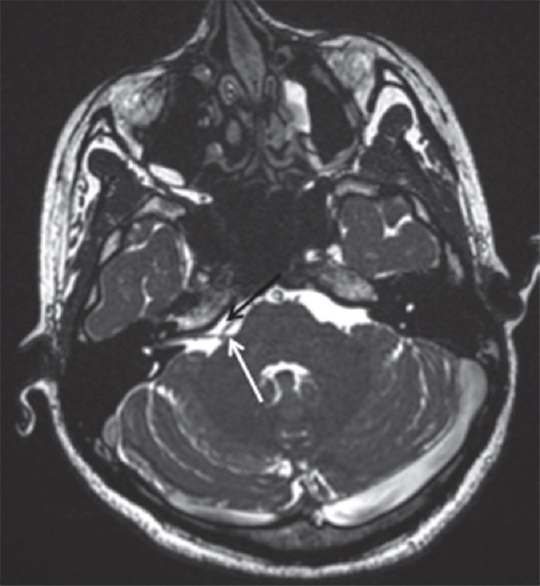
- Magnetic resonance imaging brain (three-dimensional constructive interference in steady-state sequences) showing the anterior inferior cerebellar artery (black arrow) abutting the root entry zone of the facial nerve (white arrow) on the right side with no evidence of any mass lesions
DISCUSSION
MVD has remained the gold standard surgery for the treatment of TN and HS, providing excellent long-term results.[1278] The most common complications include CSF leak, facial hypesthesia, facial paresis, chemical meningitis, and hearing loss.[12478] Delayed facial weakness is an uncommon complication, mostly reported after MVD for HS. It has also been reported after vestibular schwannoma surgery.[9] Although delayed hearing loss is occasionally noted after MVD for TN, there has been no report of DFP developing after surgical decompression for TN.[11011]
The incidence of DFP after MVD for HS ranges from 2.8% to 8.3% as quoted in various reports.[3561213] Rhee et al. noted an incidence of 5.4% (21 cases) in their series of 410 cases of MVD performed for HS.[6] In another retrospective analysis of 248 cases of MVD for HS, the authors noted an incidence of 6.5% (16 cases).[3] It is most commonly noted in the fifth to sixth decades. However, one report by Sekula et al. on MVD for HS in the elderly found that DFP was seen in only 3.7% in the elderly as compared to 11.5% in the younger cohort.[13] Although there is no sex predilection for DFP, this complication appears to be slightly more common in females as noted in a few reports. This is probably because of the simple reason that the incidence of HS per se is more common in females.[456] In one report, around 80% of those who developed DFP were females operated for HS.[6] In another report of 1354 cases by Han et al., the authors noted that incidence of DFP was 67% in females as compared to 33% in males.[5] As noted in our cases too, both were young to middle-aged females (<40 years) who developed this complication.
The onset of DFP is often relatively sudden and usually progresses over a 24-h period.[34] The average time to onset of DFP after surgery ranges from 1 week to 12 days, in various series.[3461314] The etiology of DFP remains unclear and DFP can develop even after a successful operation up to several days after MVD.[35] Hence, it is believed that direct trauma to the nerve is an unlikely cause of this complication.[4] Kim et al. opined that the possible causes include facial nerve exit zone injury through the Teflon felt or delayed facial nerve edema.[12] An ischemic hypothesis was suggested by Scheller et al., in which a microcirculation disturbance occurs due to vasospasm causing delayed paresis. Hence, they instituted postoperative vasoactive therapy with nimodipine for patients with DFP after acoustic neuroma surgery and noted that all their patients improved.[15] Neural edema has been proposed as a cause of DFP.[69] It mainly occurs at the level of the meatal foramen and plays a deleterious role at the fundus of the meatus because this is the narrowest part of the Fallopian canal.[36] Liu et al. concluded that hypertension might be a contributing factor for occurrence of DFP as noted in their series of 16 DFP cases.[3] Small hemorrhages into the facial canal (similar to retinal hemorrhages) and partial neural necrosis may explain the association between hypertension and facial palsy.[3]
A possible viral etiology in the form of reactivation of herpes simplex virus (HSV) or varicella zoster virus as a cause of DFP has been proposed by many authors.[316] This possibility of viral reactivation phenomenon is well known and has been reported after vestibular schwannoma surgery.[69] Manipulation of the nerve or nervus intermedius can stimulate a dormant virus, possibly localized in the geniculate ganglion.[34816] Furthermore, viral reactivation may be contributed by the surgery itself as it itself is a major stress on the immune system. This in turn might generate an inflammatory reaction at the geniculate ganglion level coursing through the meatal part of the nerve serving as a source of ischemia and demyelination before denervation.[3] Hence, Furukawa et al. advocated early treatment with intravenous acyclovir for DFP to avoid any evolution to neurotmesis and subsequent sequelae.[16] In one report, 44 of the 410 patients were shown to be infected with HSV after MVD; however, none of them developed DFP.[6] Similar findings were reported by Han et al.[5] Liu et al. noted that only 1 of their 16 cases of DFP had HSV infection.[3] Kuroki et al. found that not all patients with DFP were positive for the CSF IgM antibody to HSV.[14] Thus, the exact role of HSV in the causation of DFP is debatable and remains to be clarified.
Although no specific predisposing factors exist for DFP, based on the available reports, we are of the opinion that females, younger age, and hypertension are possible risk factors for the occurrence of this complication after MVD procedure. Although known to occur after acoustic tumor surgeries and MVD for HS, DFP has not been reported after MVD for TN till date. In the largest series of MVD for TN by Barker et al., the most common complications noted were chemical meningitis, CSF leak, facial numbness, facial palsy, and hearing loss, with no mention of DFP in their report.[1] Our reports show that DFP can also occur after MVD for TN. In our first case, the patient developed immediate postoperative complications in the form of sensorineural hearing loss and Grade 2 facial paresis which may be due to the cerebellar retraction during retrosigmoid approach. This might have caused minor damage to the facial nerve too, which manifested as an overt facial palsy after a delayed period. However, in the second case of HS, there was direct handling of the facial nerve in an attempt to isolate it from the vessel. It appears clear from the above cases that occurrence of DFP is related to facial nerve injury, either directly during surgical dissection or indirectly such as a stretch on the facial nerve occurring during cerebellar retraction.
Radiological investigations do not attain much priority in such cases as noted in a few reports stating that none of the cases demonstrate any abnormality.[4] Similarly, in both our cases, CT was essentially normal without any evidence of hematoma or infarct. Since DFP is an uncommon complication and resolves spontaneously in most cases, the best treatment is still not known. Multiple treatment options exist including steroids, acyclovir, and decompression of the facial nerve at the Fallopian canal.[461718] Many may recover spontaneously; hence, the role of the above treatment options is questionable. Prognosis is good to excellent in majority of cases. The average duration from onset to recovery ranges from 5.7 weeks to 9 months, in various reports. Liu et al. noted that 15/16 (95%) patients experienced a complete recovery without requiring any special treatment. They, however, noted that the time of onset correlated with the duration of DFP, i.e., an earlier development of DFP corresponded with a shorter duration, whereas a later development of DFP corresponded with a longer duration.[3] In the study by Rhee et al., the time to recovery averaged 5.7 weeks (range: 25 days – 17 weeks) and 95% patients of DFP exhibited a complete recovery without any further special treatment.[6] Sekula et al. noted that 11 out of 12 DFP cases showed complete resolution spontaneously.[13] Kuroki et al. reported that all patients had complete resolution of their delayed facial weakness.[14] Similar results were published by Hongo et al.[19] Lovely et al. noted that the time to recovery averaged 6.5 weeks (range: 1–28 weeks) and all patients improved to either complete recovery or minimal HB Grade II weakness.[4]
Steroids have been tried in a few cases that may be helpful in reducing the edema hoping to hasten recovery and a few cases have been treated with acyclovir for a possible viral etiology.[3461620] Han et al. used a combined therapy in treating a few cases of DFP. Acyclovir (30 mg/kg/day) and prednisolone (1 mg/kg/day) were given for 94 patients for at least 2 weeks. Complete recovery (Grade I) was seen in 86% and eight patients improved to minimal weakness (Grade II). The time to recovery averaged 64.1 days (range: 16–270 days).[5] In another report, prednisolone (1 mg/kg/day) was initiated and gradually discontinued over 2 weeks, depending on the symptoms. However, the exact role of steroids in improving the outcome was not clear because overall 95% of patients showed good–excellent recovery.[6] Similarly, 11 out of 28 cases received steroid medications, but all 28 cases showed complete recovery/minimal weakness on follow-up.[4] Both our cases received steroids combined with acyclovir in 1 case for 2–3 weeks duration, and the average time to recovery of DFP was 4.5 weeks from its onset. Thus, the role of steroids and acyclovir is controversial, but probably they might assist in faster recovery. However, this remains to be proven in future studies.
CONCLUSIONS
DFP is an uncommon complication occurring after MVD for HS. We also report the first case of DFP occurring after MVD for TN. The occurrence of DFP is related to facial nerve injury, either direct or indirect. It usually occurs within 2 weeks of the surgery and majority of them show good to excellent outcomes. Radiological investigations do not provide any added information. The role of viral reactivation remains debatable. Furthermore, the role of steroids and acyclovir remains to be proven; however, it might assist in faster recovery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077-83.
- [Google Scholar]
- Prognosis research of delayed facial palsy after microvascular decompression for hemifacial spasm. Acta Neurochir (Wien). 2016;158:379-85.
- [Google Scholar]
- Delayed facial weakness after microvascular decompression of cranial nerve VII. Surg Neurol. 1998;50:449-52.
- [Google Scholar]
- Delayed cranial nerve palsy after microvascular decompression for hemifacial spasm. J Korean Neurosurg Soc. 2012;52:288-92.
- [Google Scholar]
- Frequency and prognosis of delayed facial palsy after microvascular decompression for hemifacial spasm. Acta Neurochir (Wien). 2006;148:839-43.
- [Google Scholar]
- Microvascular decompression for trigeminal neuralgia in the elderly: Long-term treatment outcome and comparison with younger patients. Neurosurgery. 2009;65:477-82.
- [Google Scholar]
- Microvascular decompression to treat hemifacial spasm: Long-term results for a consecutive series of 143 patients. Neurosurgery. 2002;50:712-8.
- [Google Scholar]
- Delayed facial paralysis after vestibular schwannoma surgery: Role of herpes viruses reactivation – our experience in eight cases. Otol Neurotol. 2004;25:805-10.
- [Google Scholar]
- Delayed hearing loss after microvascular decompression of the trigeminal nerve. Acta Neurochir (Wien). 1998;140:94-7.
- [Google Scholar]
- Delayed hearing loss after neurovascular decompression. Neurosurgery. 1990;27:997-1003.
- [Google Scholar]
- Delayed facial palsy following microvascular decompression in hemifacial spasm. J Korean Neurosurg Soc. 1999;28:1332-6.
- [Google Scholar]
- Microvascular decompression for hemifacial spasm in patients >65 years of age: An analysis of outcomes and complications. Muscle Nerve. 2013;48:770-6.
- [Google Scholar]
- Delayed facial palsy after microvascular decompression for hemifacial spasm. Facial Nerve Res. 1991;11:147-50.
- [Google Scholar]
- Delayed facial nerve paresis following acoustic neuroma resection and postoperative vasoactive treatment. Zentralbl Neurochir. 2004;65:103-7.
- [Google Scholar]
- Delayed facial palsy after microvascular decompression for hemifacial spasm due to reactivation of varicella-zoster virus. No Shinkei Geka. 2003;31:899-902.
- [Google Scholar]
- On the mechanism of transient postoperative deficit of cranial nerve. Surg Neurol. 1999;51:223-6.
- [Google Scholar]
- Delayed facial palsy after acoustic neuroma resection: The role of viral reactivation. Am J Otol. 1996;17:625-9.
- [Google Scholar]
- Posterior fossa microvascular decompression for hemifacial spasm and trigeminal neuralgia – Some improvements on operative devices and technique. No Shinkei Geka. 1985;13:1291-6.
- [Google Scholar]
- Delayed cranial neuropathy after neurosurgery caused by herpes simplex virus reactivation: Report of three cases. Surg Neurol. 2005;64:67-9.
- [Google Scholar]






