Translate this page into:
Decompression Hemicraniectomy for Refractory Intracranial Hypertension in Reversible Cerebral Vasoconstriction Syndrome
Address for correspondence: Dr. Naresh Mullaguri, Division of Neurocritical Care, Cerebrovascular Center, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, Ohio 44195, USA. E-mail: mullaguri.nari@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) is a disorder of dysregulation of cerebrovascular tone resulting in transient segmental vasoconstriction which resolves in 1–3 months. Cerebral edema is an underrecognized complication in RCVS. It is likely multifactorial. This edema can lead to intracranial hypertension that can be refractory to medical management. Limited evidence exists regarding surgical management of intracranial hypertension in RCVS. We present a 29-year-old Caucasian right-handed female patient with a medical history of migraine, polysubstance abuse presented to the emergency department (ED) daily for 3 days with the chief complaint of recurrent thunderclap headache. She declined neuroimaging and lumbar puncture. She was treated for migraine with abortive medications with no improvement. During the third ED visit, she became lethargic with right-sided homonymous hemianopia. Computerized tomography of the brain showed left parietal intracerebral hemorrhage with intraventricular extension, cortical subarachnoid hemorrhage, and diffuse cerebral edema. Digital subtraction angiography showed multifocal moderate-to-severe segmental vasoconstriction suggestive of vasculopathy. Oral verapamil was initiated. Continuous intracranial pressure monitoring showed uncontrolled intracranial hypertension, despite maximal medical management with hyperosmolar therapy, induced coma, and hypothermia. Decompressive hemicraniectomy with duraplasty was performed for refractory intracranial hypertension. We provisionally diagnosed her with RCVS. She was discharged to inpatient rehabilitation with residual right homonymous hemianopia. Transcranial Doppler study during follow-up showed improved mean flow velocities. She continued to have residual cognitive deficits with complete resolution of headache.
Keywords
Decompressive hemicraniectomy
intracranial hypertension
reversible cerebral vasoconstriction syndrome
stroke
vasospasm
INTRODUCTION
Reversible cerebral vasoconstriction syndrome (RCVS) is a disorder of dysregulation of cerebrovascular tone resulting in transient segmental vasoconstriction which resolves in 1–3 months.[1] Thunderclap headache is the most common presenting symptom, followed by focal neurological deficits and seizures. The exact etiology of RCVS is unknown. Multiple risk factors have been attributed to trigger its development such as migraine, pregnancy, sympathomimetic drug exposure, triptans, ergot derivatives, selective serotonin reuptake inhibitors, blood transfusions, chemotherapy drugs, and intracranial hypotension.[12] About 30% of RCVS patients will have initial neuroimaging findings of cortical subarachnoid hemorrhage (cSAH), intracerebral hemorrhage (ICH), acute ischemic stroke (AIS), and posterior reversible encephalopathy syndrome (PRES) and dissection of cervical arteries.[1234] The angiographic characteristic of this syndrome shows “sausage on a string appearance” of vasospasm.[3] Despite the florid neuroimaging abnormalities, the prognosis is usually excellent with 98% of patients functionally independent and <5% mortality at 1 year.[5] Hemorrhagic complications, such as ICH and cSAH, occur early in the course secondary to severe vasospasm of smaller arteries, followed by progressive centripetal vasoconstriction of larger arteries leading to infarction.[56] Cerebral edema is an underrecognized complication in RCVS. It can be secondary to ischemic and hemorrhagic stroke, SAH, PRES, vasospasm with subclinical ischemia, hydrocephalus, or disruption of the blood–brain barrier.[7] Only one case report regarding the surgical management of cerebral edema in RCVS has been reported to date.[8] We describe a case of hemorrhagic RCVS with uncontrolled intracranial hypertension, despite maximal medical management requiring decompressive hemicraniectomy with good prognosis.
CASE REPORT
A 29-year-old Caucasian right-handed female with a medical history of migraine, polysubstance abuse (opioids, tetrahydrocannabinol, and methamphetamine), depression with recent suicidal ideation, posttraumatic stress disorder, and smoking presented to the emergency department (ED) with the chief complaint of holocranial headache for 1-day duration. It was sudden and severe in onset, stabbing in character, and severe (8–9/10) in intensity with periodic exacerbations with a constant low-grade background headache. She smoked half a pack of cigarettes daily and marijuana at least 2–3 times in 1 week. Physical examination showed nuchal rigidity with no focal deficits. She was diagnosed with migraine exacerbation and treated with intravenous ketorolac, promethazine, and orphenadrine. She was discharged home from the emergency room.
She returned to ED with an unremitting headache. She was again treated for migraine, including subcutaneous sumatriptan. Toxicology screen came back positive for cannabinoid and methamphetamines. She left the ED after some improvement in pain but refused additional investigations. She came back on day 3 with worsening of headache but continued to refuse neuroimaging and lumbar puncture. She came back the same evening with new-onset left-sided homonymous hemianopia and disorientation. Vitals showed blood pressure of 155/94 mm of Hg. CT scan of the brain showed large acute to subacute left parietooccipital ICH (50 cm3) with intraventricular extension and 1 cm left-to-right midline shift at the frontal horn of the lateral ventricle along with cortical and tentorial SAH. It also showed diffuse cerebral edema, obstructive hydrocephalus with effacement of ambient and quadrigeminal cisterns [Figure 1a and b]. Her blood work showed leukocytosis of 17,220/uL with neutrophilic predominance and elevated D-dimer at 2760 ng/ml. Other coagulation laboratories were unremarkable at presentation. No deep vein thrombus was found in a duplex ultrasound scan of the lower extremities. The exact cause of elevated D-dimer was unknown but presumed due to ICH. She was transferred to neurointensive care unit for further management.
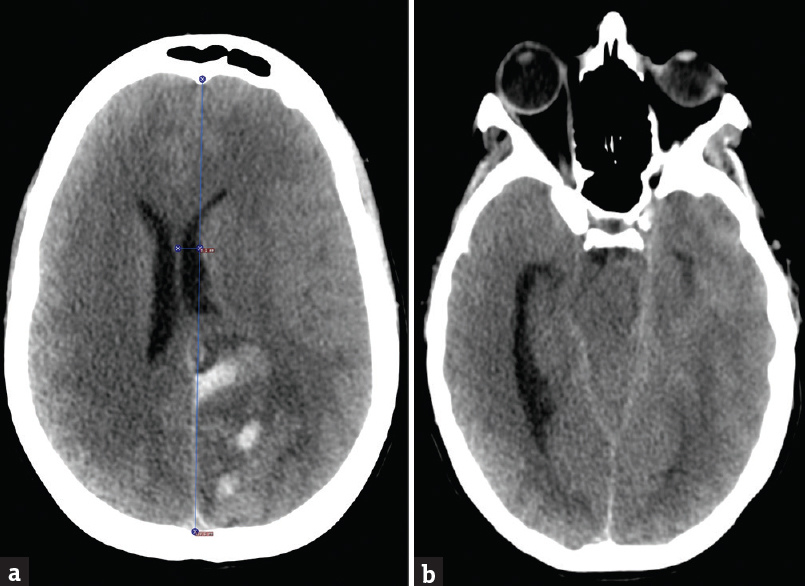
- Computerized tomography of the brain axial sections; (a) section through frontal horns of the lateral ventricles showing left parietooccipital hemorrhage with a left-to-right midline shift and diffuse cerebral edema. (b) Section through the temporal horn of the lateral ventricle showing obstructive hydrocephalus with trapped left-sided temporal horn and severely dilated the right temporal horn. The section also shows effacement of quadrigeminal and basal cisterns concerning for impending herniation
She was started on levetiracetam for seizure prophylaxis and nimodipine for vasospasm prophylaxis from SAH. Computerized tomography (CT) angiogram of the head on the 4th day from ictus showed multiple areas of narrowing in the bilateral anterior cerebral arteries (ACAs) and distal left posterior inferior cerebellar artery with no aneurysms, vascular malformations, or spot sign; neck angiography was unremarkable. Digital subtraction angiography confirmed multifocal, moderate-to-severe segmental narrowing in anterior and posterior circulation predominantly in the ACA, posterior cerebral arteries [Figure 2a], and basilar artery. Venous phase of angiography did not show venous sinus thrombosis. Cerebrospinal fluid (CSF) analysis showed 136 mg/dL protein, 24 nucleated cells/uL with 91% neutrophils, 93 mg/dL of glucose, and 7000/uL red blood cells. CSF Gram stain and culture were negative. HIV, fungal cultures, and syphilis tested were negative. Erythrocyte sedimentation rate, C-reactive protein, and autoimmune and systemic vasculitis panels were negative. She was provisionally diagnosed with RCVS. She was started on oral verapamil and gradually uptitrated to prevent hypotension (40 mg every 8 h) for headache and continuous intravenous fentanyl infusion for headache. A repeat CT brain on the 5th day showed worsening cerebral edema with stable ICH. She was intubated due to a worsening level of consciousness, and a left frontal bolt was placed. Intracranial pressure (ICP) was in the range of 40–50 mm of Hg. She was started on hyperosmolar therapy with hypertonic saline. Serum sodium increased to 162 mmol/L, and hyperosmolar therapy was stopped. She continued to have high ICP. She was sedated and paralyzed with continuous atracurium (train of four 0–2/4), continuous propofol, fentanyl, and midazolam infusions. Electroencephalogram monitoring was initiated and achieved burst suppression. Targeted temperature management was initiated (36° centigrade) (Arctic Sun, BARD, California). Despite maximal medical management, her ICP was not controlled (maximum 47 mm of Hg). After discussing with family, left-sided decompressive hemicraniectomy with duraplasty (DHD) and a brain biopsy was performed [Figure 2b]. A right frontal external ventricular drain (EVD) was placed, and bolt was removed. The biopsy was negative for inflammation and vasculitis. On day 7, she was weaned off of hypothermia, sedatives, and paralytics. Daily bedside transcranial Doppler (TCD) studies showed moderate vasospasm [Table 1]. We stopped daily TCDs given improving clinical examination. On day 10, her episodes of thunderclap headache resolved, and EVD was removed. She was extubated on day 12 and was discharged to acute inpatient rehabilitation facility with residual right homonymous hemianopia (modified Rankin score of 2). TCD study at 2 months’ follow-up visit showed normal mean flow velocities [Table 1]. Cranioplasty was performed after 3 months with no complications. She continued to have psychomotor slowing and memory difficulties with complete resolution of homonymous hemianopia and headaches at 90 days.
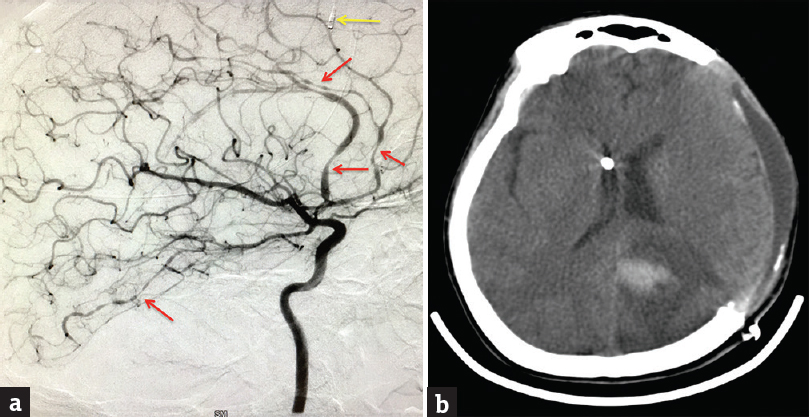
- (a) Digital subtraction angiography of the brain, selective left internal carotid artery injection showing multifocal segmental vasoconstriction of the anterior cerebral and posterior cerebral arteries (red arrows). Left frontal lobe parenchymal intracranial pressure probe is also seen (yellow arrow). (b) Computerized tomography of the brain axial section showing left-sided parietooccipital intracerebral hemorrhage with decompressive hemicraniectomy and external herniation. A right frontal external ventricular drain is also seen
| Insonated intracranial artery (MFV and PI) | Day 7 | Day 8 | Day 9 | Day 65 |
|---|---|---|---|---|
| Right MCA | 171 cm/s; 0.82 | 145 cm/s; 0.89 | 166 cm/s; 0.85 | 103 cm/s; 0.91 |
| Left MCA | 138 cm/s; 0.95 | 145 cm/s; 0.91 | 139 cm/s; 0.78 | 97 cm/s; 0.97 |
| Right ACA | 74 cm/s; 1.07 | 87 cm/s; 0.87 | 93 cm/s; 0.72 | 59 cm/s; 0.93 |
| Left ACA | 78 cm/s; 0.82 | 81 cm/s; 1.08 | 119 cm/s; 0.89 | 68 cm/s; 0.95 |
| Right PCA | 64 cm/s; 0.89 | 52 cm/s; 0.89 | 57 cm/s; 0.73 | 52 cm/s; 1.0 |
| Left PCA | 47 cm/s; 0.96 | 79 cm/s; 0.70 | 39 cm/s; 1.2 | 32 cm/s; 0.99 |
| Basilar artery | 97 cm/s; 0.64 | 98 cm/s; 1.02 | 104 cm/s; 0.91 | 67 cm/s; 1.0 |
MCA: Middle cerebral artery, ACA: Anterior cerebral artery, PCA: Posterior cerebral artery, MFV: Mean flow velocities, PI: Pulsatility Index
DISCUSSION
Cerebral edema is an underrecognized complication in RCVS and possibly multifactorial in etiology. Decompressive hemicraniectomy can be life-saving treatment for refractory intracranial hypertension in RCVS patients that can result in a good prognosis.
RCVS is mostly monophasic, noninflammatory vasculopathy due to the failure of cerebral autoregulation. The proposed criteria for RCVS must have acute and severe thunderclap headache, cSAH, multivessel segmental vasoconstriction on angiography, relatively normal CSF, and resolution of vasoconstriction on follow-up angiography in 12 weeks. Some have proposed the initial response of intra-arterial vasodilator therapy response to diagnose RCVS.[9] Although the exact etiology is unknown, many risk factors are attributed to RCVS in the literature, including serotonergic, sympathomimetic, immunosuppressive drugs, postpartum state, blood transfusions, and intracranial hypotension.[10] ICH, PRES, and cSAH occur early in the course of RVCS as in our patient, followed by AIS changes concurrent with the centripetal progression of the vasospasm from distal cortical arteries to proximal vessels at the circle of Willis, respectively.[23] Isolated cortical vasogenic edema and hyperintense vessel signs were described as early signs of RCVS in a study by Murase et al.[11] Blood–brain barrier disruption, oxidative stress, increased F2-isoprostanes secretion, and cerebral endothelial dysfunction are reported as possible mechanisms for vasoconstriction.[7111213] There is no effective cure for RCVS, but fortunately, it is a self-limiting, benign vasculopathy with good prognosis. Up to 5% of patients will be significantly disabled or dead due to ICH, AIS complicated by severe vasospasm, cerebral edema, and intracranial hypertension. Calcium channel blockers such as verapamil were shown to be beneficial. Triptans and glucocorticoids (given for recurrent migraine or suspicion of the central nervous system angiitis, respectively) can lead to poor prognosis.[1415] Intra-arterial vasodilator drugs, such as nimodipine, verapamil, and milrinone, have also been used with variable success along with angioplasty for severe vasospasm.[1916]
Hemorrhagic RCVS occurs in one-third of RCVS patients and some with associated diffuse cerebral edema due to multifocal ICH, cSAH, and PRES changes.[6] The initial ICH burden predicts the outcome and thus preventing secondary brain injury from intracranial hypertension is of paramount importance to minimize disability. Singhal et al., in their pooled analysis of 139 cases, found 9% of patients had unfavorable outcome (modified Rankin Scale 4–6) with 2% mortality due to severe vasoconstriction. Vasoconstrictive drugs were shown to be associated with severe cerebral edema in the study by Singhal et al., similar to our case.[2] Interestingly, ischemic stroke predicted poor outcome rather than ICH. This may be secondary to longer duration of severe vasoconstriction in those patients. TCD at bedside is a valuable noninvasive tool to monitor vasospasm similar to subarachnoid hemorrhage patients.[17] Reversibility of vasospasm in serial TCD examinations may point toward the diagnosis of RCVS instead of other inflammatory vasculopathies.[17] We adopted a similar strategy to diagnose RCVS in our patient.
Mechanism of refractory cerebral edema in RCVS is poorly understood. The diffuse cerebral edema in our patient can be attributed to multiple factors such as female sex, methamphetamine abuse, ICH, and cSAH with superimposed PRES changes as described in the prior studies.[2] Conservative management with hyperosmolar therapy, sedation, paralysis, and targeted temperature management are usually the strategies for the management of cerebral edema irrespective of its etiology. Medical management of cerebral edema in RCVS patients is challenging due to unknown optimal blood pressure targets, limitations of hyperosmolar therapy, and lack of studies on targeted temperature management given the simultaneous hemorrhagic and ischemic complications. Despite hyperosmolar therapy, Paralytics, sedation, and therapeutic hypothermia, we were unable to control her intracranial hypertension forcing us to pursue surgical options. DHD was a life-saving measure in the management of refractory intracranial hypertension. Given the benign nature and excellent prognosis of RCVS, DHD might improve functional outcome as in our patient.
CONCLUSIONS
Hemorrhagic RCVS patients can present with refractory intracranial hypertension. Decompressive hemicraniectomy is safe and may be considered in patients with uncontrolled intracranial hypertension despite maximal medical management. Prospective randomized studies are needed to confirm the benefit of hemicraniectomy in hemorrhagic RCVS patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Narrative review: Reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34-44.
- [Google Scholar]
- Reversible cerebral vasoconstriction syndromes: Analysis of 139 cases. Arch Neurol. 2011;68:1005-12.
- [Google Scholar]
- The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:3091-101.
- [Google Scholar]
- The link between migraine, reversible cerebral vasoconstriction syndrome and cervical artery dissection. Headache. 2016;56:645-56.
- [Google Scholar]
- Long-term outcomes after reversible cerebral vasoconstriction syndrome. Cephalalgia. 2016;36:387-94.
- [Google Scholar]
- Progressive manifestations of reversible cerebral vasoconstriction syndrome presenting with subarachnoid hemorrhage, intracerebral hemorrhage, and cerebral infarction. J Korean Neurosurg Soc. 2014;56:419-22.
- [Google Scholar]
- Blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome: Implications for pathophysiology and diagnosis. Ann Neurol. 2017;81:454-66.
- [Google Scholar]
- Reversible cerebral vasoconstriction syndrome with intracranial hypertension: Should decompressive craniectomy be considered? Case Rep Neurol. 2017;9:6-11.
- [Google Scholar]
- A novel approach to diagnose reversible cerebral vasoconstriction syndrome: A case series. J Stroke Cerebrovasc Dis. 2015;24:e31-7.
- [Google Scholar]
- The typical thunderclap headache of reversible cerebral vasoconstriction syndrome and its various triggers. Headache. 2016;56:657-73.
- [Google Scholar]
- Isolated cortical vasogenic edema and hyperintense vessel signs may be early features of reversible cerebral vasoconstriction syndrome: Case reports. Cephalalgia. 2018;38:1207-10.
- [Google Scholar]
- Oxidative stress and increased formation of vasoconstricting F2-isoprostanes in patients with reversible cerebral vasoconstriction syndrome. Free Radic Biol Med. 2013;61:243-8.
- [Google Scholar]
- Cerebral vasomotor reactivity in reversible cerebral vasoconstriction syndrome. Cephalalgia. 2017;37:541-7.
- [Google Scholar]
- The need for a rational approach to vasoconstrictive syndromes: Transcranial Doppler and calcium channel blockade in reversible cerebral vasoconstriction syndrome. Case Rep Neurol. 2016;8:161-71.
- [Google Scholar]
- Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology. 2017;88:228-36.
- [Google Scholar]
- Intra-arterial milrinone for reversible cerebral vasoconstriction syndrome. Headache. 2009;49:142-5.
- [Google Scholar]
- Transcranial Doppler ultrasonography as a non-invasive tool for diagnosis and monitoring of reversible cerebral vasoconstriction syndrome. R I Med J (2013). 2016;99:38-41.
- [Google Scholar]






