Translate this page into:
Crude Extracts of Bacopa Monnieri Induces Dendrite Formation in Rodent Neural Stem Cell Cultures—A Possible Use in Neuronal Injury
Rohit Kumar Sarda, MSc Department of Anatomy, Sikkim Manipal Institute of Medical Science 5th Mile Tadong, Gangtok, Sikkim 737102 India rohit.s@smims.smu.edu.in
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Repair of nervous tissue injury impairs positive functional outcome. Major challenges involved are formation of new neuronal cells at the site of injury, growth and development of existing or stem cell-derived neuronal cells, and proper anatomical alignment of the cells required for the functional organization of the nervous system. Stem cells and various agents have been tried to overcome the above challenges yielding only limited positive results. Bacopa has been in frequent usage for cognitive impairment in Ayurvedic medicine. The assumption that Bacopa monnieri (BM) extracts may lead to certain specific changes at the cellular structural level benefitting the central nervous system repair, prompted us for the present study.
Objective This is an in vitro study evaluating the effect of BM extracts (bacopasides and analogues) on the neuronal stem cells (NSC) culture in various concentrations. The study investigates the possibility of BM as an agent for the regeneration and differentiation of nervous tissue injury. This may have clinical and therapeutic implications.
Materials and Methods NSC were harvested from the newborn albino rats, Rattus norvegicus, and the BM extracts were obtained from product “brahmi” manufactured by Himalaya Drug Company. Aqueous suspension of 2 μL of alcoholic extract of BM was locally added to the culture plates of NSC in concentrations of 5, 10, and 20 µg/mL after development of NSC in the media. The control NSC (without BM) and BM-rich NSC were simultaneously observed at regular unit intervals after inoculation. The morphological change in the NSC were observed and recorded.
Result NSC could be successfully cultured from the newborn rat's brain harvested at 3 and 6 hours of birth. NSCs derived at 3 hours of birth were more primitive (predominantly neurospheres) than derived those at 6 hours of birth. BM had significant positive effect on the neurospheres, that is, dendritic formation was seen in the NSC predominating when 2 μL of suspensions containing 5 and 10 µg/mL concentration of the extracts were used but showed relatively lesser effect at concentration of 20 µg/mL. The positive effect was biologically significant.
Conclusion NSC can be cultured from brain of the newborn rodent. BM and its extracts act positively on NSC in terms of dendritic formation when used in proper appropriate concentration. The study opens up a new area of research and explores newer avenues in nervous tissue injury repair. It may have future clinical implication in the treatment of injury of central and peripheral nervous tissue. However, the hypothesis needs to be validated by adequate number of experimental runs as well as in vivo studies to know the reproducibility of the findings in other centers.
Keywords
neural stem cells
Bacopa
nerve regeneration/drug effects
medicine
Ayurveda
Introduction
Recovery from injury of the central nervous system (CNS) is grossly limited because of inadequate capacity to regenerate. Functional deficits following CNS injury results from mechanical insult leading to demyelination, axonal damage, and loss of glia and neurons. The damaging effects are further pronounced due to further secondary processes like ischemia, anoxia, free radical formation, and excitotoxicity. The solution seems to be possibly in the regenerative capacity of the neuronal or/other stem cells at the site of injury. Neural progenitor cells (NPCs) isolated from the adult CNS differentiate into neurons and glia after transplantation into the brain. They also differentiate into oligodendrocytes and astrocytes after transplantation into the spinal cord.1 Even rat and human mesenchymal stem cells retain the capacity to differentiate into nonmesenchymal derivatives, specifically into neurons.2 Embryonic stem cells derived oligodendrocytes have been shown to produce myelin in vitro and after transplantation are capable of myelinating axons in vitro in injured CNS.3 Human and rat CNS stem cells grow as neurospheres (CNS-stem cells) and are capable of surviving and differentiating without contributing to the glial scars.4 An agent that can facilitate the differentiation of these stem cells into the mature nerve cells promoting anatomical and functional organization at the injured site shall be highly beneficial for the CNS recovery. Bacopa Monnieri (BM), a herb belonging to the family Scrophulariaceae and commonly known as “Brahmi” in India, has been implicated to have beneficiary role in improving learning and memory in animals and humans.5 6 7 BM extracts help to repair damaged neurons and exhibit neuroprotective effect against oxidative stress in the hippocampus of the rat brain.8 The neuroprotective effects of BM may be attributed to its ability to suppress neuronal oxidative stress and also to its acetylcholinesterase inhibitory activities.9 We hypothesized that since BM has neuroprotective effect it may have a significant role in the differentiation of the neuronal stem cells (NSCs) leading to significant morphological changes which may contribute to recovery from CNS injury. Hence, we designed a preliminary in vitro experiment to study the effect of BM extracts on indigenously cultured NSC. In this study, NSCs were cultured from the brain of newborn rat and once the neurospheres were evident they were divided into control group (NSC-CON) and BM incorporated (NSC-BM) group. The NSC-BM group was subjected to the different concentrations of BM extracts. The results were analyzed based on the morphological changes in the NSCs. It was found that the BM extract had a positive effect on the dendritic formation of the NSC which was biologically significant. This study provides for an alternative approach to repair of traumatized neural tissue. It may have future implication in the central and peripheral nervous tissue treatment.
Materials and Methods
The study is a preliminary in vitro experimental study evaluating the role of BM on the dendritic formation of NSC. The study involved isolation of NSCs, expansion, culture, and differentiation of isolated stem cells, preparation of BM in various concentrations, inoculation of the prepared BM in the stem cell culture, and comparative assessment of the various groups. The study was approved and cleared by the Institutional Ethical Committee and Animal Ethical Committee (vide letter no. MC/SMIMS/IAEC/01/2015).
Isolation of Neonatal Neuronal Stem Cells
NSCs were isolated from the brain of newly born albino laboratory rodents (Rattus norvegicus) at 3rd and 6th hours after birth. The rodents were procured from the animal house of Sikkim Manipal Institute of Medical Sciences. Neonatal rodents less than 6 hours old were brought to the research laboratory and sterilized by wiping the whole body twice with 70% alcohol and then by keeping under ultraviolet rays for 20 minutes in laminar flow. The rodents were euthanized by decapitation and skin with scalp was removed by surgical blades (No. 15). Skull bone was removed and dura with brain was exposed. Brain was chipped out with blades in small pieces and kept in a Petri dish with phosphate-buffered saline (PBS) solution. Brain was then macerated with blade to fine homogenous mixture and then washed by transferring the material to 2 mL conical centrifuge tubes (Eppendorf) and centrifuged for 2 minutes at 300 × g (1,380 revolutions per minute). Supernatant was decanted carefully and then fresh PBS solution was poured and mixed thoroughly. The fresh solution was again spun for 2 minutes at 300× g and supernatant was decanted carefully. This freshly prepared media solution was poured into the Eppendorf and mixed thoroughly. Viable cell count was done with using Trypan blue solution in hemocytometer.
Media Preparation for Expanding Rat Neonatal Neural Stem Cells
The media for expanding the stem cells was prepared as per instructions in the kit. The StemProNSF SFM 100 mL solution was prepared with 1 × 97 mL of KnockOut D-MEM/F12 (Invitrogen, United States), 2 mL of 2% StemPro neural supplement (Invitrogen), 1 mL of 2 mM GlutaMAX- I Supplement (Invitrogen), 20 µL of 20 µg/mL basic fibroblast growth factor (bFGF) (Invitrogen), 20 µL of 20 µg/mL epidermal growth factor (EGF) (Invitrogen), and 1 mL antibiotics to make total solution volume of 100 ml.
Differentiating Media
Differentiating media was used for allowing BM to help neurospheres to differentiate into nerve cells. This media did not have growth factor in them and the rest of the media was same as complete StemProNSF SFM without bFGF and EGF.
Preparation of Bacopa Monnieri (Brahmi) for Inoculation
Dry powder form of BM “brahmi” 250 mg/capsule (Himalaya Drug Company). Stock solution of BM was made by mixing contents of one capsule of BM (250 mg) thoroughly with 1,000 mL of distilled water. Sonication was done for 2 minutes to mix the product in distilled water by ultrasonic homogenizer (Hieshler, model UP 100 H). The solution was filtered with microsyringe filter and sterilized with microspore filter size 45 μm. The required concentration of 5, 10, and 20 µg/mL was prepared from stock solution. Standardizing of Bacopa was done using Agilent QTOF liquid chromatography system.
Culture and Inoculation of BM
The culture of rat NSCs was done from the cell solution prepared from the isolated cells harvested from brain of newborn rat. About 200 µL of cell suspension was plated on uncoated culture flask (200 mL) containing 20 mL of complete StemProNSF SFM media and antibiotic solution. The culture bottle was incubated in CO2 incubator (New Brunswick, model: Galaxy -40R) at 37°C with 5% CO2 and 95 to 98% of humidity for 24 hours. The culture which showed neurospheres at 96 hours were further centrifuged and cells were taken out in StemProNSF SFM media without growth factors, that is, bFGF and EGF. The fresh culture was suspended in the flask and then taken in cell viewer culture plates in duplicates. The culture plates were marked as BM 5 µg/mL, BM 10 µg/mL, and BM 20 µg/mL. Each plate was inoculated with 200 µL of media and 200 µL of cell suspension. Thus, NSC-CON (without BM) and NSC-BM (with BM) at 5, 10, and 20 µg/mL groups were created. Note that 2 µL of BM solution at various concentrations were inoculated in the respective culture plates and all groups were incubated. Phase microscopy was done at 24, 48, 72, and 96 hours using fluorescence Leica Microscope DMIL – LED FLUO. Photography was done at each time by the inbuilt digital camera DFC 295 and the results were assessed.
Results
Neuronal Stem Cell can be Harvested from Newborn Rodent Whole Brain Irrespective of Area Specificity
NSC was isolated from the rodent brains at 3 and 6 hours of birth. The newborn rodent brain could yield a sufficient number of viable NSC which could be successfully cultured (Fig. 1). NSC harvested from the rat brain was first cultured with growth factors (bFGF and EGF) but without BM. The NSC on culturing with the growth factors formed dendrites after 48 hours in both the groups. It was found that at 72 hours, NSC obtained at 6 hours of birth showed more evident dendritic formation as compared with NSC harvested at 3 hours of birth (Fig. 2). Both the groups developed dendrite by 96 to 120 hours. It was inferred that the NSCs can be harvested and cultured from the newly born rat's brain and the NSCs obtained at 6 hours of birth were more mature considering the extent of dendritic formation. This finding indicated the regenerating capacity of newly born rat's brain due to presence of stem cells. This regenerating capacity shall presumably be higher at earlier age in lieu of presence of more primitive stem cells.
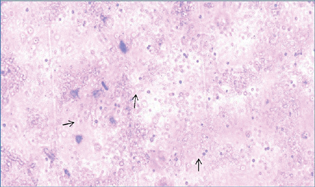
-
Fig. 1 Viable neuronal stem cell (black arrow) obtained from the newborn rat's brain.
Fig. 1 Viable neuronal stem cell (black arrow) obtained from the newborn rat's brain.
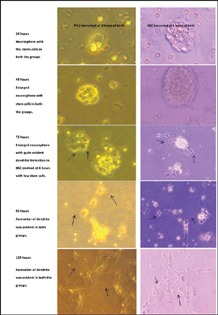
-
Fig. 2 Formation of dendritic formation (black arrows) of the neuronal stem cell cultured without Bacopa Monnieri in presence of growth factors.
Fig. 2 Formation of dendritic formation (black arrows) of the neuronal stem cell cultured without Bacopa Monnieri in presence of growth factors.
Bacopa Monnieri has a Positive Effect on Neuronal Stem Cell Growth and Dendrite Formation
The NSC formed neurospheres after 24 hours of culture (Figs. 2 and 3). The formation of neurospheres was better appreciated in BM-instilled specimens in comparison to the control group. The BM-instilled cultures (NSC-BM) formed dendrite in 24 hours, irrespective of the concentration; but the control (NSC-CON) formed dendrite only after 48 hours. The extent and size of dendrite formation in NSC-CON was less than the NSC-BM group even at 72 to 120 hours. The difference in these characteristics of the dendrites was biologically significant in NSC-BM in comparison to NSC-CON. The concentration variation of the BM also played a significant role on the dendrite formation. The concentration of 5 and 10 µg/mL had relatively better positive effect than 20 µg/mL concentration. A significant difference due to the concentration was appreciated at 120 hours of culture (Fig. 3). However, no toxic effect was noticed at the used concentrations of 5, 10, or 20 µg/mL. BM thus acted like a catalytic agent in promoting neuronal growth and formation of dendrite. The arborization pattern was also evident at 120 hours of the culture.
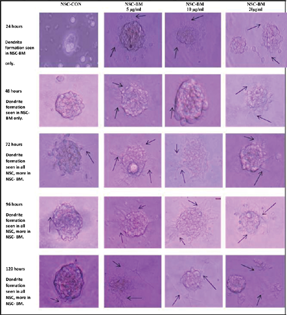
-
Fig. 3 Formation of dendrites (black arrow) and effect of Bacopa Monnieri (BM) at 5, 10, and 20 µg/mL on newborn (at 3 hours of birth) rat's brain-derived neuronal stem cell (NSC-BM) in comparison to neuronal stem cells without BM (NSC-CON).
Fig. 3 Formation of dendrites (black arrow) and effect of Bacopa Monnieri (BM) at 5, 10, and 20 µg/mL on newborn (at 3 hours of birth) rat's brain-derived neuronal stem cell (NSC-BM) in comparison to neuronal stem cells without BM (NSC-CON).
Discussion
The existence of adult CNS multipotent neural stem cells has opened a new avenue for the growing field of cell therapy as a means to repair CNS injuries and provides a platform to explore stem cell-based therapy in CNS injury repair.10 Several studies have been performed to evaluate the possibility of presence of multipotent NSC in adult CNS and their capability in treating neurological diseases. Fetal CNS stem cells transplant have shown clinical improvement in Parkinson's patients. Transplant of fetal CNS tissue has been performed in human patients with Parkinson's disease, resulting in some clinical improvement.11 12 13 Transplantation of brain-derived adult NPCs is an effective strategy to replace oligodendrocytes and promote the remyelination of surviving axons after spinal cord injury.14 Ependymal cells have been proposed as rapidly proliferating cells which generate neurons which can differentiate astrocyte and participate in scar formation.15 The spinal cord epidermal region-derived stem/progenitor cells have the ability to self-renew and of multipotentiality for neurons and glia.16 In most of the researches a lot of focus has been on generation of NSCs from embryonic tissue and placental cord tissue. Subventricular tissue in adult mammalian brain has been implicated to be the source of neural stem cell.17 Neurogenesis has been believed to end after few days of life but Reynolds and Weiss10 demonstrated that adult mouse striatum cells have the capacity to divide and differentiate into neurons and astrocytes. The present in vitro study explored the feasibility of harvesting NSC from the newborn rat's brain within few hours of birth. This study successfully demonstrates the possibility of generating NSC from the newborn rat's whole brain irrespective of area specificity in the laboratory set up. However, the multipotentiality needs to be studied in detail. The study helps in establishing the hypothesis that NSC thus derived can be differentiated into neurons with dendrites. In vitro generated NSC can possibly play a significant role in managing neurological disorders. In vitro expanded NSCs have been transplanted in spinal cord injury and shown to repair the CNS with neurogenesis and provide functional recovery.18
In vitro culturing of NSCs is feasible in the presence of growth factors like FGF, EGF, and others. An agent that can replace or supplement the existing factors shall be highly beneficial for the neuronal regenerative treatment. The present study evaluated the efficacy of the traditional Ayurvedic Indian medicine namely BM. It is a traditional medicinal herb found in India, China, Nepal, Sri Lanka, and Taiwan and has been recommended for treatment of various mental disorders. Studies show BM extracts to be nontoxic, nonteratogenic, and nonmutagenic in rats and monkeys.19 20 A longer period BM extract treatment in rats with higher dose brought about induced structural changes in basolateral amygdaloid neurons which went on to improve learning and memory.21 Brahmi (local Indian name of BM) antagonize the effect of scopolamine in mice thus improving cognitive functions of brain.22 It has also been found to enhance wound healing23 and have a broad spectrum of antibacterial activity.24 Dried whole plant of BM with 50% ethanol extract decrease free radical levels, promoting antioxidant status, and has anxiolytic effect, antidepressant activity, and anticonvulsive action.25 26 BM extracts help to repair damaged neurons and show neuroprotective effect against oxidative stress in the hippocampus of the rat brain.8 BM extracts have also been used for treatment of neonatal hypoglycemia.27 Treatment with BM extracts significantly reduced lipid peroxidation, lipofuscin deposition, and attenuated structural derangements in the hippocampus.21 Considering extensive positive effect of the BM extracts on neuronal cells, the present study was planned with the novel idea of checking its ability in in vitro setup. In the present study, higher doses of BM (20, 40, and 80 mg/kg) over long duration of 4 to 6 weeks have shown dendritic formation in Wister rats' basolateral amygdala neurons.21 The present study demonstrated positive effect at low doses like 5, 10, and 20 µg/mL in the culture media, with the best response at dose between 5 and 10 µg/mL. Further studies need to be done in animals to see if the same effects are present at the plasma levels of BM extract at concentration of 5 and 10 µg/mL. It remains to be ascertained which oral dossing would achieve such plasma levels. The dendritic formation was evident after 24 hours of culture and significant changes were found after 120 hours of culture. BM has been earlier implicated for exhibiting neuroprotective effects on oxidative stress, but the present study hints toward its role in neuron growth and differentiation. The structural changes due to BM extracts in the neurons have also been earlier delineated with electron microscopic evaluation.28 The present study also demonstrated structural changes that were beneficial in the in vitro neuronal growth and differentiation. It is noteworthy that even in the absence of growth factors, BM had positive effect on neuronal growth and differentiation. Detail evaluation of BM and its extracts is needed to ascertain the exact mechanism resulting to the beneficiary structural changes.
Conclusion
The brain of newborn rats contains NSCs that can be harvested and cultured. The stem cells are more primitive at earlier age. As seen in tissue culture, BM, used in proper concentration (5–10 µg/mL), has a positive effect on NSC derived from the newborn rat's brain in terms of dendritic formation. The feasibility of BM as an agent for neuronal growth and differentiation can be further explored. The study opens up a new area of pharmacologic usage in nervous tissue injury repair and neuronal regeneration. It may have future clinical implication in central and peripheral nervous tissue disorder treatment especially using stem cells.
However, study is limited by the following issues. It is a preliminary study and the result can only be validated after adequate number of further experimental runs required for confirmation of the hypothesis. Further, the positive effect of BM needs to be ascertained on the NSCs derived from other sources. The result may have to be evaluated in the in-vivo set up or actual clinical conditions of nervous tissue repair.
Acknowledgment
The authors acknowledge the team members of the research group, institute research unit, ICMR, and Himalayan Drug Company for their generous help in the respective areas.
Conflict of Interest
None declared.
References
- Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92(25):11879-11883.
- [Google Scholar]
- Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364-370.
- [Google Scholar]
- Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;97(11):6126-6131.
- [Google Scholar]
- Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102(39):14069-14074.
- [Google Scholar]
- Effect of Bacopa monniera Linn. (brahmi) extract on avoidance responses in rat. J Ethnopharmacol. 1982;5(2):205-214.
- [Google Scholar]
- Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn.(Brahmi) Indian J Pharmacol. 1997;29(5):359.
- [Google Scholar]
- Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology. 2006;27(4):451-457.
- [Google Scholar]
- Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008;120(1):112-117.
- [Google Scholar]
- Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710. (5052):
- [Google Scholar]
- Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. N Engl J Med. 1992;327(22):1549-1555.
- [Google Scholar]
- Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson's disease. Ann Neurol. 1995;38(3):379-388.
- [Google Scholar]
- Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990;247:574-577. (4942):
- [Google Scholar]
- Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26(13):3377-3389.
- [Google Scholar]
- Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25-34.
- [Google Scholar]
- Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 2007;24(5):835-845.
- [Google Scholar]
- Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703-716.
- [Google Scholar]
- Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69(6):925-933.
- [Google Scholar]
- Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12(4):305-317.
- [Google Scholar]
- Enhancement of basolateral amygdaloid neuronal dendritic arborization following Bacopa monniera extract treatment in adult rats. Clinics (São Paulo). 2011;66(4):663-671.
- [Google Scholar]
- Activity of antioxidant enzymes in the skin during surgical wounds. Bull Exp Biol Med. 2006;142(6):667-669. English, Russian.
- [Google Scholar]
- Potential antimicrobial activity of various extracts of Bacopa monnieri (Linn.) Int J Pharmacol. 2008;4(3):230-232.
- [Google Scholar]
- Anxiolytic profile of standardized Brahmi extract. Indian J Pharmacol. 2000;32:152.
- [Google Scholar]
- Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine. 2002;9(3):207-211.
- [Google Scholar]
- Neuroprotective potential of Bacopa monnieri and Bacoside A against dopamine receptor dysfunction in the cerebral cortex of neonatal hypoglycaemic rats. Cell Mol Neurobiol. 2013;33(8):1065-1074.
- [Google Scholar]
- Neuroprotective effects of Bacopa monniera whole-plant extract against aluminum-induced hippocampus damage in rats: evidence from electron microscopic images. Chin J Physiol. 2014;57(5):279-285.
- [Google Scholar]







