Translate this page into:
Cranioplasty Using Autoclaved Autologous Skull Bone Flaps Preserved at Ambient Temperature
Address for correspondence: Dr. Rajeev Aravindakshan, Department of Community Medicine, Pushpagiri Institute of Medical Sciences and Research Centre, Thiruvalla, Kerala, India. E-mail: rajeevtka@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Decompressive craniectomy followed by cranioplasty (CP) uses autologous craniectomy flaps or synthetic materials like titanium. Sterilization and preservation methods for the autologous bone flaps continue to be the surgeon's choice.
Aim:
This study aimed to assess the short-term as well as long-term clinical outcomes of CP using autoclaved autologous bone grafts.
Settings and Design:
This retrospective observational study was performed on patients admitted in a tertiary care teaching neurosurgery department.
Patients and Methods:
Seventy-two patients who underwent CP with autoclaved autologous skull flaps preserved under ambient conditions with strict aseptic precautions were included in the study.
Statistical Analysis Used:
Frequencies and percentages of the various characteristics before and after the surgery were tabulated. Continuous variables were summarized as means and standard deviations.
Results:
The primary CP had a satisfactory clinical outcome in 62 cases (86.11%). Osteomyelitis was observed in four patients (5.56%) nearly 2 months after the surgery. Radiologically significant bone resorption was noted in a single patient (1.39%) after 1 year. Five patients (6.94%) developed bone fragmentation or fracture, and the mean time taken for this was about 36 months. In all these ten cases, secondary CP was successfully done using a prefabricated, patient-specific titanium mesh.
Conclusions:
The efficacy and safety of the studied craniectomy flaps used for cranial reconstruction showed a good patient outcome. Further retrospective studies with larger cohorts and prospective case–control studies are essential so as to issue standard guidelines for sterilization and preservation of autologous bone flaps.
Keywords
Ambient temperature
autoclaving
autologous skull bone flap
cranioplasty
storage
INTRODUCTION
Cranioplasty (CP) following decompressive craniectomy (DC) by itself has a relatively higher postoperative complication rate than other cranial interventions.[12] The simplest method of CP is grafting the same bone flap that was removed during craniectomy. There are a lot of controversial observations regarding sterilization and storage of these skull bone flaps prior to cranial reconstruction. Many centers preserve the sterilized bone flaps in deep freezers, and thaw them just before CP.[1] In the current series, we used autologous bone grafts, cleaned and autoclaved soon after craniectomy, stored at ambient temperatures with sterile precautions, and autoclaved again a day prior to CP.
Aim
This study aimed to assess the short-term as well as long-term clinical outcomes of CP using autoclaved autologous bone grafts.
PATIENTS AND METHODS
This study was conducted at the department of neurosurgery of a tertiary care teaching hospital in Central Kerala, India. The study population was 72 patients subjected to CP using autologous bone flaps, following DC for various reasons, from November 2008 to October 2015. The patient records were studied retrospectively and followed up for about 3 years. Patients of all age groups requiring CP following DC where skull flap was surgically removed and preserved as one piece, under ambient temperature, ensuring meticulous sterile precautions, were included in this study. Exclusion criteria were patients who had craniectomy for tumours or having a history of previous CP, who had perioperative sepsis in craniectomy or infection or fragmentation of the bone flap and complex fractures. Patients who were lost to follow-up for a minimum of 1 year were also excluded from the analysis.
Methodology
The present study is a retrospective, single-center, observational study, done on 72 consecutive patients who underwent primary CP with the autoclaved autologous skull flaps as per informed consent process approved by the hospital management. A total of 86 patients had undergone CP during this period, but 14 of them were left out as per the exclusion criteria.
The DC was done in majority of cases as a standard fronto-temporo-parietal hemicraniectomy, and the dura was opened in a stellate manner. Lax duroplasty was done using a dural substitute and the scalp was closed in two layers.
The craniectomy bone flaps debrided of soft tissues were washed thoroughly with betadine solution to make them clean of blood and fibrofatty tissue. They were sealed in sterilization pouches in two layers and autoclaved at 132°C for 20 min. The sterilized flaps were stored within the same pouch, within sealed boxes, in cupboards, at ambient temperature. On the day prior to the date of CP, these were autoclaved again in the same manner. The sterile pouch was then opened and the bone flap was placed overnight in platelet-rich plasma solution harvested from the patient's blood.
At CP, the scalp layers were incised open, corresponding to the previous incision. Temporalis muscle was dissected off the dura carefully to avoid a cerebrospinal fluid (CSF) leak. The edges of the skull at the craniectomy site were trimmed using a drill so that the cranial defect and the bone graft corresponded exactly. The bone flap was fixed to the skull using titanium miniplates and screws. To cover the defects corresponding to burr hole, or gap between the bone edges, if any, reinforcement with titanium mesh was done. The temporalis muscle and scalp were closed in layers. Subgaleal drain was inserted before wound closure and retained for 48 h, and intravenous antibiotics were administered for 7 days postoperatively.
All patients were assessed clinically and by computed tomography (CT) scan soon after the CP and just before discharge, and then followed up in the outpatient department for not <2 years. Patients complaining of softening of bone flaps or unacceptable cosmetic results were subjected to CT scan. Presence of osteolytic lesions in both inner and outer tables of the graft, or obvious thinning of the bone, without clinical or hematological signs of infection, was considered as bone flap resorption (BFR). Surgical site infection (SSI) is generally described as infection resulting in surgical removal of the implanted graft.
Problems encountered during the first 7 days after surgery were considered early complications, and those that occurred after the 7th day were considered late complications. Early complications looked for include discharge from the wound site, wound dehiscence, implant displacement, seizures, intracranial hematoma, and associated systemic problems such as pneumonitis and deep vein thrombosis. The CP was considered failed when the cranium had to be re-opened for a secondary CP using an artificial graft.
The data were coded into a spreadsheet and the analysis was carried out. Frequencies and percentages were calculated for the categorical variables and tabulated or plotted. The continuous variables were summarized into means and standard deviations.
RESULTS
Over a 7-year period from November 2008 to October 2015, 72 patients underwent primary CP using preserved autologous bone graft. Of these, fifty patients were male and 22 were female. There were two children; a female child aged 13 years and a male child of 11 years. The mean age of the patients was 40.28 (± 15.9) years; seven patients were aged over 60 years.
The indication for the previous DC [Figure 1] was noted in all patients, the most common being traumatic brain injury. The time interval between the DC procedure and the CP was highly variable (0–60 weeks), with a mean period of 12.82 (± 9.26) weeks in the present study.
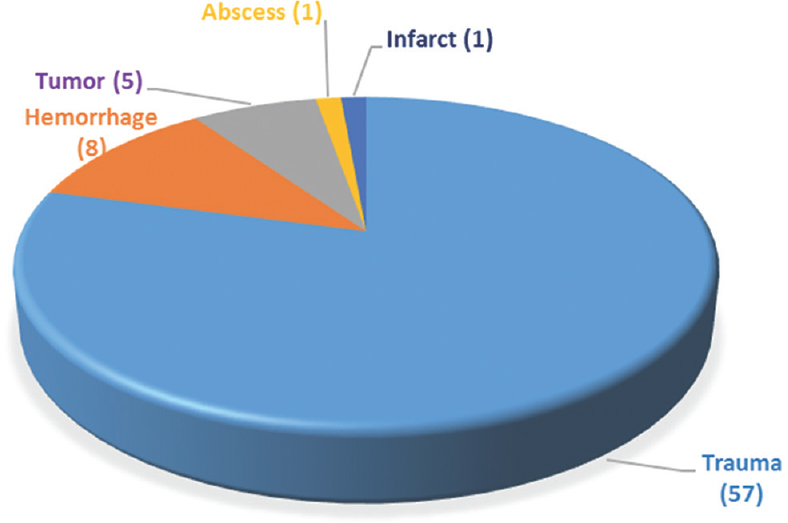
- Indications for decompressive craniectomy
CP was required bilaterally in four patients where it was bi-frontal [Table 1], and was unilateral in the rest, with fronto-temporo-parietal being the most common, with slight left-side preponderance. The duration of hospital stay required for CP was observed to be variable, between 4 and 45 days, with a mean period of 9.08 (± 4.81) days. The mean follow-up period, post-CP, was about 18 months.

The most relevant clinical parameter studied was Glasgow Outcome Score; its mean values before and after CP were 4.22 (± 0.58) and 4.33 (± 0.94), respectively. The postoperative period was totally uneventful and recovery was satisfactory in 37 patients. Mild surgical wound infection with a discharging sinus was noted in three patients, and this could be successfully managed with antibiotics as per culture and sensitivity reports. Wound dehiscence was not observed in any patient. Aspiration had to be done in almost half of the study group, once in 25 patients, twice in 11 patients, and six times in one patient. Some cases of recurrent collections were managed by inserting a Mini Vac® suction drain which was kept in situ till the drainage became negligible. Epidural/subdural hematoma occurred in three patients, which was managed by drill hole tapping. Mild intracerebral hemorrhage occurred in one patient, which subsided spontaneously under observation. Seizures were noted in 23 patients, which settled with routine anticonvulsant medications. Pneumonitis occurred in four patients and deep vein thrombosis in two patients. The bone flaps were not found to be displaced in any patient.
Late complications ultimately ended up as failed CP. Indications for secondary CP included osteomyelitis, BFR, and fragmentation or fracture. In our study, infection in the form of osteomyelitis was met with in four patients (5.56%), within a period of nearly 2 months after the surgery. Radiologically significant bone resorption [Figure 2a] was noted only after 1 year, and this was seen in a single case (1.39%), demanding further intervention. Five patients (6.94%) developed bone fragmentation or fracture [Figure 2b], and the mean time period taken for this was about 36 months. In all the above ten cases, secondary CP was done using a prefabricated, patient-specific titanium mesh. Mild BFR was seen in nine patients but did not call for any active intervention, being cosmetically acceptable. Hydrocephalus was encountered in two patients and they were treated successfully by inserting a ventriculoperitoneal (VP) shunt.
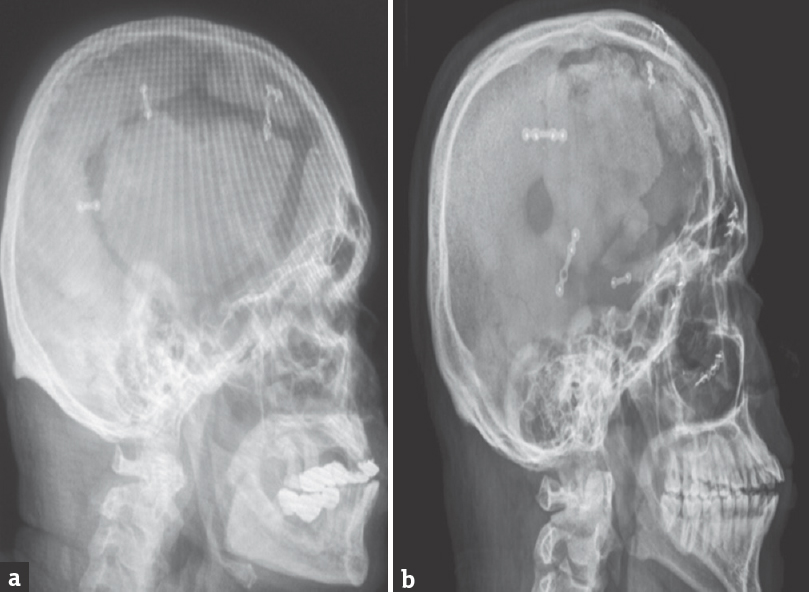
- (a) Bone flap resorption along the edges of the graft. (b) Bone flap fragmentation/fracture
DISCUSSION
A variety of materials are being used for repairing skull defects, grouped into autografts, allografts, and bone substitutes. The success and durability of the cranial reconstruction demanded careful selection of the material tailored to the individual's requirements. The ideal material would be sterilizable, nonmagnetic, radiolucent, light weight, and easily secured to the skull. The bone flap removed during craniectomy is generally considered adequate for reconstructive surgery as it is viable, corresponds exactly to the cranial defect, and has good potential for growth and low rate of fragmentation or migration, as opined by Zhang et al. At the same time, the long-term grave complications, i.e., BFR, SSI, and bone fragmentation could contribute to significant patient morbidity.[1]
The present study did not reveal any appreciable influence of patient age or sex on the clinical outcome, and there was no major difference in the size of the cranial defect among the various patients. Rocque et al. also reported that age of the patient or size of the defect does not influence the patient outcome.[2] However, Lee et al. in a detailed study on the resorption of autogenous bone graft reported a higher resorption rate for larger bone flaps.[3]
The period of delay in doing CP after craniectomy showed gross difference among various studies, possibly due to the varied preferences of the surgeons. The mean interval between the two procedures in the current series of patients of our institution was 12.82 weeks (varying from 0 to 60 weeks). A study of 61 pediatric cases of autologous CP by Piedra et al. observed that resorption was significantly more common in the late CP group (42%) than that of the early CP group (14%). However, no other complication showed significant difference between the two cohorts.[4] A study of bone resorption in delayed cranioplasties using cryopreserved autologous skull flaps done by Brommeland et al. showed the median time between DC and CP as 74 days (19–353 days).[5]
Effect of the time lag between craniectomy and CP was studied in a systematic review by Rocque et al.[2] The authors of three out of the four articles studied found no significant effect, but one article claimed that bone resorption was lower in children who underwent early CP. They also noted that freshly harvested autografts have lower rate of resorption than that of stored autografts. In a case series of 82 patients, Thavarajah et al. suggested that the CP procedure should be delayed as much as 6 months to minimize the risk of SSI.[6] Rocque et al. specifically looked for infection among sixty patients implanted with fresh autograft from hip bone or adjacent calvaria, but not a single case of infection was observed. However, infections occurred in 16 out of 214 surgeries (7%) using stored bone flaps.[2] Yadla et al.[7] found no significant difference in the infection rates of early (earlier than 3 months) versus delayed (later than 3 months) surgery; this was confirmed by Wachter et al.[8] as well.
Studies on pediatric patients undergoing the same surgical procedures have been reported in a few articles.[591011] Martin et al. felt that this age factor could be due to normal calvarial growth, with dynamic remodeling and osteoclastic mechanisms, making the flap vulnerable to osteolysis.[9] Grant et al. in a study on failed cranioplasties reported BFR in twenty out of forty patients below the age of 19 years.[10] They observed BFR in a mean period of 13.3 months. Furthermore, Bowers et al.[11] demonstrated BFR as high as 81.8% in pediatric CP. Brommeland et al. reported that four out of five patients below the age of 15 years developed osteolysis after autologous CP, and underwent revision CP.[5] The number of patients in the pediatric age group in the present study was too small for a significant observation, but yet, the two candidates, having a gap of 12 weeks and 20 weeks between the two surgeries, respectively, took up the autologous grafts well, with no sign of BFR after follow-up of 5 years.
The methods of extracorporeal storage of bone flaps vary widely, the most frequent method reported is cryopreservation at −16°C to −86°C.[15121314] The complication rates appear to vary with the freezing temperatures. There are many reports of preserving the bone flaps in ordinary refrigerator (−8°C to 8°C).[15] Reports of bone flaps being stored in ambient conditions are also available.[2161718]
Rocque et al. in a pediatric study using cryopreserved bone concluded that it may cause higher rate of bone resorption and lower rate of infection compared to room temperature storage.[2] In a systematic review, they looked into BFR and SSI in 441 patients, obtained from 11 original studies. Of these, six studies used cryopreserved bone and one had bone flap preserved at ambient temperature. In this sole study by Josan et al., the bone flaps were autoclaved and stored in sterile packets at ambient temperature.[16] None among these 12 patients in this group reportedly developed BFR. Compared to cryopreserved graft, the incidence of resorption was lower, and this was statistically significant (P = 0.003). The incidence of infection was noted by the same authors to be statistically significantly higher (P = 0.103) in the bone flaps stored in ambient conditions.
Kim et al. looked for risk factors that could cause BFR in 129 patients, all bone flaps being stored at ambient temperatures.[17] Indications for craniectomy were traumatic brain injury (56), cerebral infarction (15), subarachnoid hemorrhage (30), intracerebral hemorrhage (15), and miscellaneous (13). BFR had a relatively highest incidence in subarachnoid hemorrhage, probably due to an already compromised blood flow to the bone tissue, resulting from atherosclerosis.
In a study on the long-term complications of CP, Schuss et al. observed that 60% of BFR occurred within 1 year of follow-up, and no resorption occurred after a period of 5 years.[19] Piedra et al. found that a longer freezing time of 6 weeks[20] led to an increase in BFR from 14% to 42%. Cryopreserved bone (−80°C) was used by Brommeland et al. in 87 patients, of which 31 (36%) had morbid complications and 22 lost their primary implant.[5] SSI and BFR were common, affecting 8 (9.2%) and 14 (19.7%) patients, respectively. However, 73% of patients had successful reinsertion of autologous graft at affordable costs. The authors suggested synthetic implants for pediatric patients, fragmented bone flaps, and delayed CP.
Mracek et al. used autoclaved autologous bone flaps stored in ordinary refrigerator at 8°C. They observed such 110 patients for more than 2 years and concluded that operating time and diabetes mellitus are independent risk factors for SSI.[15] So also, the presence of VP shunt was stated to be an independent risk factor in the development of BFR. According to Zhang et al., the siphon action of the VP shunt could lead to fine motion of the bone flap and increased bone resorption.[1] Moreover, this fine motion might cause titanium screw loosening and fixation failure. However, such a problem was not encountered in the present study where two cases had VP shunting done for hydrocephalus.
As reported by Hng et al., a total of 187 patients had autologous bone flaps cryopreserved at −30°C, and the common complications occurred were infection (11.2%) and bone resorption (5.35%). In addition, they pointed out that intraoperative CSF leak was significantly associated with infection, whereas longer duration of surgery was associated with bone resorption.[12]
Sundseth et al. studied 74 patients, of which 66% had cryopreserved bone flap which were retrieved from refrigerator only when the patient reached the operation theater.[13] The bone was then placed in gentamicin–NaCl solution. Six patients developed SSI, and the bone flaps had to be removed 5, 17, 39, 119, 164, and 729 days after CP. The authors also observed that no statistically significant association existed between SSI and prophylactic antibiotics or postoperative subcutaneous drainage.
Ozaki in a clinical and experimental study on frozen bone grafts observed that autoclaving the bone before implantation seemed to reduce the risk of SSI, but aggravate the risk of BFR.[14]
Some methods of sterilization of stored skull bone flaps are autoclaving, ethylene oxide (EtO), plasma (gaseous H2O2), and gamma irradiation. The high incidence of infection following CP necessitates optimal sterilization methods, but presently no standard guidelines are available, so it still remains the surgeon's choice.
Zhang et al. rinsed the removed bone flaps with physiological saline, placed in sterile gloves, and immersed in 0.5% povidone-iodine solution and then placed into a storage bag for cryopreservation at −20°C.[1] EtO gas sterilization and ambient temperature storage of autologous bone were done in 103 patients by Jho et al.[18] Cranioplasties were performed about 4 months later and the follow-up averaged 14 months. Excellent results were achieved in 95 patients (92.2%); however, SSI occurred in eight patients (7.8%). They observed that preservation beyond 10 months was associated with an increased risk of SSI. The authors propose this sterilization method for any institution that provides EtO gas sterilization services.
Kim et al. dried the cleaned bone flap in a warming cabinet at 110–120°C for 1–2 days and then sterilized at 70°C for 75 min with H2O2 and low temperature gas plasma.[17] The flap was then stored aseptically at ambient temperature, and re-sterilized similarly twice more, 1 and 2 days before CP, with acceptable results. Schuss et al. found BFR more frequently in patients with multiple fractures or fragments in the bone flap than in those without fragmentation (17.2% versus 2.2%, respectively). They observed that the larger surface area of bony fragments in multiple fractures makes bone proteins more susceptible to denaturation during sterilization and impair osteogenesis.
SSI in our study was 5.56%, considerably lower than the incidence in most of the (7%–11%) other reported studies.[21218] This observation justifies the sterilization and preservation methods used in our study.
CONCLUSIONS
Autologous skull bone flap is probably the best material available for CP following DC. Autoclaving is an effective and safe method of sterilization of such skull bone flaps, and storage of the sterilized bone flaps under ambient temperature gives a good patient outcome. Prospective studies on CP appear grossly insufficient and should be conducted on large patient groups for statistically significant observations. Comparative studies are required so as to arrive at a consensus regarding the best methods of sterilization and preservation of autologous bone flaps.
Limitations of the study
This was a retrospective study from a single institution, and not prospective. There was no control group for comparison between the various methods of bone flap sterilization and preservation. The number of patients in pediatric and adolescent age groups was too small for any relevant conclusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Cranioplasty with autogenous bone flaps cryopreserved in povidone iodine: A long-term follow-up study? J Neurosurg 2017:1-8. doi: 10.3171/2016.8.JNS16204 [Epub ahead of print]
- [Google Scholar]
- Outcomes of cranioplasty following decompressive craniectomy in the pediatric population. J Neurosurg Pediatr. 2013;12:120-5.
- [Google Scholar]
- Resorption of autogenous bone graft in cranioplasty: Resorption and reintegration failure. Korean J Neurotrauma. 2014;10:10-4.
- [Google Scholar]
- Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr. 2012;10:268-72.
- [Google Scholar]
- Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med. 2015;23:75.
- [Google Scholar]
- The minimum time for cranioplasty insertion from craniectomy is six months to reduce risk of infection – A case series of 82 patients. Br J Neurosurg. 2012;26:78-80.
- [Google Scholar]
- Effect of early surgery, material, and method of flap preservation on cranioplasty infections: A systematic review. Neurosurgery. 2011;68:1124-9.
- [Google Scholar]
- Cranioplasty after decompressive hemicraniectomy: Underestimated surgery-associated complications? Clin Neurol Neurosurg. 2013;115:1293-7.
- [Google Scholar]
- Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in paediatric traumatic brain injury patients. Acta Neurochir (Wien). 2014;28:34-9.
- [Google Scholar]
- Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004;100:163-8.
- [Google Scholar]
- Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr. 2013;11:526-32.
- [Google Scholar]
- Delayed cranioplasty: Outcomes using frozen autologous bone flaps. Craniomaxillofac Trauma Reconstr. 2015;8:190-7.
- [Google Scholar]
- Cranioplasty with autologous cryopreserved bone after decompressive craniectomy: Complications and risk factors for developing surgical site infection. Acta Neurochir (Wien). 2014;156:805-11.
- [Google Scholar]
- Clinical and experimental study for cranioplasty with autogenous frozen bone graft. J Wakayama Med Soc. 1994;45:217-25.
- [Google Scholar]
- Complications of cranioplasty using a bone flap sterilised by autoclaving following decompressive craniectomy. Acta Neurochir (Wien). 2015;157:501-6.
- [Google Scholar]
- Bone flap resorption following cranioplasty after decompressive craniectomy: Preliminary report. Korean J Neurotrauma. 2015;11:1-5.
- [Google Scholar]
- Ethylene oxide gas sterilization: A simple technique for storing explanted skull bone. Technical note. J Neurosurg. 2007;107:440-5.
- [Google Scholar]
- Bone flap resorption: Risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma. 2013;30:91-5.
- [Google Scholar]
- Timing of cranioplasty after decompressive craniectomy for ischemic or hemorrhagic stroke. J Neurosurg. 2013;118:109-14.
- [Google Scholar]






