Translate this page into:
Craniopharyngioma causing bilateral vision loss 4 months after unremarkable magnetic resonance imaging of the brain
Address for correspondence: Dr. Curtis E. Margo, Department of Ophthalmology, University of South Florida, 12901 Bruce B. Downs. Blvd., MDC Box 21, Tampa, FL 33612, USA. E-mail: cmargo@health.usf.edu
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 65-year-old man developed bilateral vision loss 4 months after magnetic resonance imaging demonstrated no lesion in the vicinity of the optic chiasm, hypothalamus, and suprasellar tissues. Repeat computed tomography 3 months later showed a predominantly cystic mass of the suprasellar cistern with extension into the anterior third ventricle, which histologically was a craniopharyngioma. The clinical course of this case fuels the controversy whether craniopharyngiomas arise from embryonic rests or can be acquired. From a clinical perspective, it raises questions about when to obtain imaging studies dedicated to the chiasm and the appropriate interval in which a scan should be repeated to exclude structural causes of bilateral vision loss.
Keywords
Bilateral vision loss
craniopharyngioma
magnetic resonance imaging
Introduction
The cellular origin of craniopharyngioma is controversial. The bimodal age distribution that peaks in childhood and in adults between the ages of 40 and 70 years supports both the theory that tumors can arise from embryonic rests of squamous cells or through an acquired process of squamous metaplasia of cells normally found in or near the adenohypothysis.[1] We describe a 65-year-old man who developed a clinically symptomatic craniopharyngioma 4 months after magnetic resonance imaging (MRI) had shown no anatomic abnormalities in the region of Rathke's pouch.
Case Report
A 65-year-old man with past history of diabetes mellitus, carotid artery disease, coronary artery atherosclerotic heart disease, sleep apnea, and hypertension was referred for evaluation of decreased vision of the left eye for 4 weeks. Four months earlier, he had MRI and magnetic resonance angiography for evaluation of dizziness, which had shown no anatomic abnormalities in the sellar region. Nor were any abnormalities of the hypothalamus, suprasellar region, or anterior third ventricle detected [Figure 1]. Dizziness resolved spontaneously. On examination, visual acuity was 20/40 OD and 20/80 OS; there was no afferent pupillary defect but color vision was decreased in both eyes, and central scotomas were found with red color test objects in both eyes. Relevant findings on ophthalmoscopy were temporal pallor of both nerves. Satisfied that the recent MRI showed no pathological lesion, no further neuroradiological studies were obtained. Laboratory evaluation revealed a normal complete blood count, serum folate, and serum vitamin B12. Serological test for syphilis and urine heavy metal screen were negative. Three months later the patient developed a new headache and vision had progressively worsened to counting fingers OU. Computed tomography (CT) showed a 2.5 cm predominantly cystic mass centered in the suprasellar cistern with extension into anterior third ventricle [Figure 2a and b]. Endoscopic subtotal resection revealed a papillary craniopharyngioma [Figure 2c]. The patient was referred to radiation therapy.
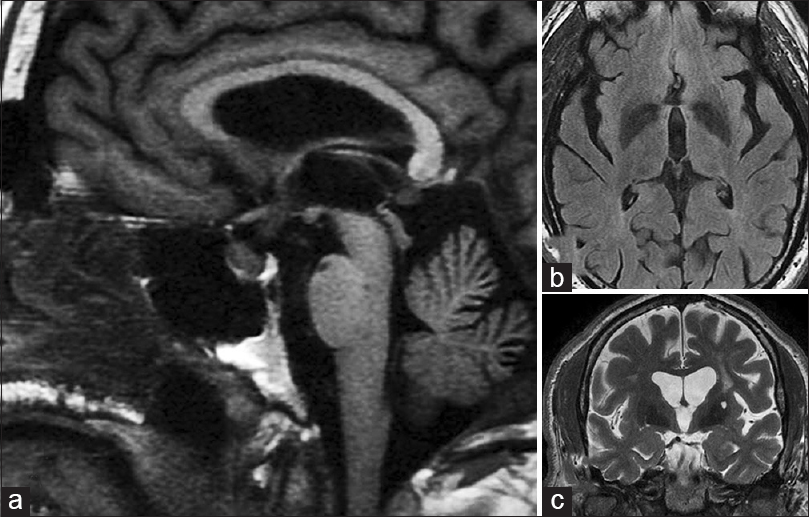
- (a) Sagittal T1-weighted magnetic resonance imaging (MRI) in the midline showing normal sellar and suprasellar structures. There is mild thickening of the pituitary stalk but no evidence of a mass (this thickening was appreciated 7 months later in retrospect). (b) Fluid attenuation inversion recovery image just rostral to the chiasm and optic nerves showing no lesions in or around anterior third ventricle. (c) A coronal T2-weighted MRI at the level of the basilar artery (arrow) just posterior to the chiasm showing no evidence of a mass (a = left; b = upper right; c = lower right)
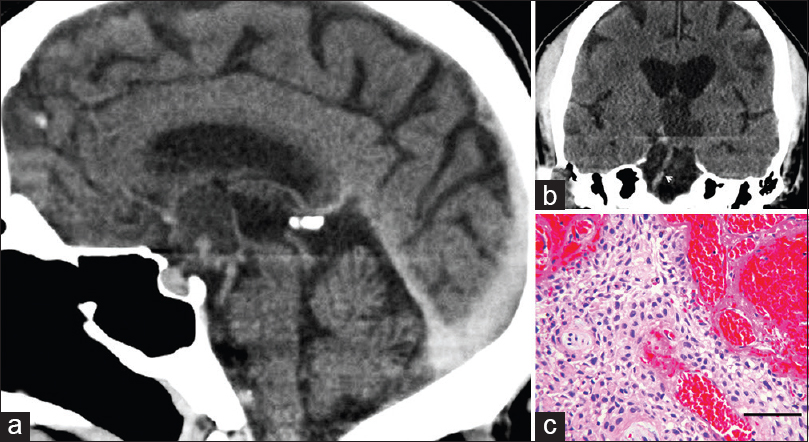
- (a) Computed tomogram 7 months after magnetic resonance imaging study shown in Figure 1. Sagittal reconstruction shows cystic mass (arrows) in the suprasellar cistern. (b) Coronal reconstruction at the level of the basilar artery (arrow) demonstrates cystic mass. The lesion is slightly hyperdense to cerebral spinal fluid in the third ventricle. (c) Microscopic examination of the partially removed tumor demonstrated sheets of squamous epithelium with peripheral palisading and intervening fibrovascular connective tissue. No mitotic figures were seen (H and E, bar = 80 microns) (a = left; b = upper right; c = lower right)
Discussion
There are 2 previous reports of craniopharyngioma arising in adults after normal neuro-radiologic studies. One occurred in a 39-year-old woman whose diagnosis was made during pregnancy.[2] She had a normal MRI 4 years earlier. The other developed in a 55-year-old man 2 years after a normal MRI.[3] These cases in addition to ours suggest that some tumors may arise de novo. Our patient sustained an injury to the visual pathway within 4 months of an unremarkable imaging study of the brain. Based on experience with the size most craniopharyngiomas obtain to deform the chiasm and become symptomatic, it was believed that the initial study sufficiently excluded a mass lesion in the suprasellar region.[4] This assumption may not have been justified.
Craniopharyngiomas are described as slow growing tumors.[5] A rare report of malignant transformation has appeared in the literature, but the vast majority of craniopharyngiomas show no significant cytological pleomorphism. The perception that most craniopharyngiomas undergo slow protracted growth prior to primary clinical detection is supported by observations of low mitotic activity and low Ki-67 labeling indicies.[67] Tumors with reportedly rapid growth or enlargement occur in the context of recurrent lesions, some of which have been reported following growth hormone replacement.[89] Long-term growth hormone replacement does not appear to be an important risk factor for recurrence, however, compared to such factors as residual tumor and initial radiation therapy.[10] More germane to this case, however, is the rate of growth prior to surgical treatment. The early natural history of craniopharyngiomas is essentially unknown since therapeutic interventions usually follow the initial discovery.
Several mechanisms may explain the presumably rapid enlargement of the tumor in our patient. It is possible that the enlarged pituitary stalk in the initial MRI (but only appreciated in retrospect) harbored a small cystic craniopharyngioma. Its enlargement could have occurred due to spontaneous rupture of the cyst or from secondary hemorrhage. The keratinaceous or colloid-like material released from cysts can induce an inflammatory response, which could simulate primary tumor growth. The biopsy from our patient showed no evidence of reactive gliosis or foreign body reaction, however, but was associated with some focal hemorrhage. Alternatively, the accumulation of extracellular material within cystic spaces can result in tumor expansion in the absence of cellular proliferation.
Visualizing structural lesions using neuroradiological imaging always involves a trade-off between resolution and signal-to-noise ratio. Although imaging technologies are constantly improving the spatial resolution, the possibility that a small tumor existed when this patient was first studied cannot be excluded. In retrospect, the initial study may not have been adequate to exclude a small compressive lesion. Given the clinical setting and knowledge of how rapidly a partially cystic craniopharyngioma can enlarge, another MRI dedicated to the chiasm with contrast should have been obtained. Because the signal intensity of tumors may blend with the signal intensity of the suprasellar cistern on T2-weighted studies, small craniopharyngiomas large enough to obliterate the suprasellar cistern can be missed.
It remains unclear at what interval neuro-imaging should be repeated when the clinical examination suggests a compressive lesion but a recent study was unremarkable, the potential difficulty of visualizing a small solid-cystic craniopharyngiomas in the suprasellar cistern must be kept in mind. We recommend reviewing the recent neuro-imaging study to ensure the sellar-chiasm was adequately visualized. If not, then a repeat study dedicated to this region, either MR with gadolinium or CT scan with contrast, should be obtained. In the context of unexplained bilateral vision loss, close monitoring for a sellar mass is mandatory even when unremarkable results are obtained on earlier scans.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Craniopharyngioma presenting during pregnancy 4 years after a normal magnetic resonance imaging scan: Case report. Neurosurgery. 2001;49:1014-6.
- [Google Scholar]
- Craniopharyngioma arising de novo in middle age. Case report. J Neurosurg. 1997;86:1046-8.
- [Google Scholar]
- Optic chiasm distortions caused by craniopharyngiomas. Clinical-MRI correlation and influence on visual outcome. World Neurosurg. 2015;83:500-29.
- [Google Scholar]
- Craniopharyngioma: A pathologic, clinical, and surgical review. Head Neck. 2012;34:1036-44.
- [Google Scholar]
- Proliferative activity in craniopharyngiomas: Clinicopathological correlations in adults and children. Surg Neurol. 2000;54:241-7.
- [Google Scholar]
- MIB-1 immunoreactivity in craniopharyngiomas: A clinico-pathological analysis. Clin Neuropathol. 2003;22:229-34.
- [Google Scholar]
- Rapid recurrence of craniopharyngioma following recombinant human growth hormone replacement. J Neurooncol. 2010;100:321-2.
- [Google Scholar]
- Rapid enlargement of a residual craniopharyngioma during short-term growth hormone replacement. Childs Nerv Syst. 2002;18:164-5.
- [Google Scholar]
- Tumour recurrence and enlargement in patients with craniopharyngioma with and without GH replacement therapy during more than 10 years of follow-up. Eur J Endocrinol. 2012;166:1061-8.
- [Google Scholar]






