Translate this page into:
Contingent negative variation for assessment of neurological deficits in children with hypothyroidism
*Corresponding author: Dr. Naveen Ravi, Senior Resident, Department of Physiology, All India Institute of Medical Sciences, Bibinagar, Hyderabad, India. drnawei@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ravi N, Bharshankar RN, Maheshwari M, Thakre AE, Wakode SL, Krishna S, et al. Contingent negative variation for assessment of neurological deficits in children with hypothyroidism. J Neurosci Rural Pract. 2024;15:557-65. doi: 10.25259/JNRP_255_2024
Abstract
Objectives:
Hypothyroidism is one of the most common endocrine disorders. Its effect on the central nervous system is more pronounced, especially in the pediatric age group. Despite receiving adequate thyroid hormone replacement therapy, several patients continue to suffer from neurological impairments including cognitive dysfunction. Contingent negative variation (CNV) is an event-related potential (ERP) that is considered as an indicator of cognitive function. In this study, CNV was recorded in children with hypothyroidism. To the best of our knowledge to date, there have been no studies of CNV in hypothyroidism.
Materials and Methods:
This prospective study included 52 children between 8 and 15 years of age who were newly diagnosed with hypothyroidism. Diagnosis of hypothyroidism was based on laboratory thyroid function tests. CNV ERP was recorded in the enrolled children at the time of diagnosis, 1-month, and 6-month follow-up. Initial CNV (iCNV) and late CNV (lCNV) amplitudes and latencies were recorded each time.
Results:
Although the amplitudes of iCNV and lCNV appeared to increase during follow-ups, the changes were not statistically significant (P > 0.05). Similarly, there appeared to be a modest reduction in latencies of iCNV or lCNV during follow-up; however, these changes were not statistically significant either.
Conclusion:
Our study did not show any significant changes in neurophysiological parameters. This may be attributed to a shorter time period of follow-up of six months and a smaller sample size. There is a possibility that CNV parameters may show more pronounced changes after a prolonged duration of treatment.
Keywords
Contingent negative variation
Hypothyroidism
Children
INTRODUCTION
Hypothyroidism is one of the most common endocrine disorders affecting millions of individuals globally and India has a very high prevalence.[1,2]
Hypothyroidism affects almost all the systems of the human body; however, the effect on the central nervous system has the most pronounced impact, more so in the pediatric age group.[1,3]
Thyroid hormones play an exquisite role in the expression of genes encoding for mitochondrial proteins,[4] in the regulation of neurofilament gene expression,[5] in the distribution of laminin,[6] and in the regulation of synapses.[7]
Congenital hypothyroidism is one of the most common causes of treatable and reversible mental retardation worldwide.[8]
Neurophysiologic tests have been performed in individuals with hypothyroidism. In them, these tests have facilitated clinical decision-making and have even impacted patient outcomes. The vast majority of these neurophysiological tests have been evoked potentials studies such as the visual, auditory, and somatosensory evoked potential studies. These evoked potentials are generally considered as indicative of cognitive function.[9]
Contingent negative variation (CNV) is an event-related neurophysiological study, wherein slow negative brain waves are recorded on an electroencephalogram (EEG) when the subject is exposed to two consecutive stimuli that are contingent on each other. CNV reflects processes such as attention, awareness, planning, decision-making, anticipation, motor preparation, time perception, and sensorimotor integration among others.[10]
Studies show that CNV amplitudes are significantly reduced in heavy alcohol drinkers,[10] individuals with post-traumatic stress disorders,[11] in multiple sclerosis,[12] Huntington’s disease,[13] end-stage renal disease,[14] and anorexia nervosa.[15]
Patients with migraine were found to have increased CNV amplitudes.[16]
In this study, we wanted to measure CNV amplitudes and latencies in children who were newly diagnosed with hypothyroidism and follow them up over a six-month duration to see if there were any changes in CNV after treatment.
Upon literature search, we were not able to find any studies pertaining to CNV in hypothyroidism.
MATERIALS AND METHODS
Study design and setting
This was a prospective study that was carried out at the Department of Physiology, All India Institute of Medical Sciences (AIIMS), Bhopal. The study was approved by the Institutional Human Ethics Committee and the Thesis Review Committee of AIIMS, Bhopal. The study was carried out over a period of 18 months.
Aims and objectives
The aims and objectives of this study are to determine the role of CNV in the assessment of neurological deficits in children with hypothyroidism; to measure the amplitude and latencies of initial CNV (iCNV) and late CNV (lCNV) in children with hypothyroidism at the time of diagnosis, 1-month, and 6-month follow-up; and to study the pattern of CNV recorded at baseline and compare it with the recordings obtained at 1-month and 6-month follow-up.
Participants
We recruited children, both boys and girls who were newly diagnosed with hypothyroidism from the Department of Paediatrics, AIIMS, Bhopal, for the study.
The inclusion criteria of this study included children of either gender or between 8 and 15 years of age who were newly diagnosed with hypothyroidism.
The exclusion criteria for this study were children with neuropsychological illnesses such as mental retardation, cerebral palsy, attention-deficit hyperactivity disorder, and learning disabilities; children with visual defects or auditory deficits, congenital hypothyroidism, and any chronic medical conditions, and children with a history of cranial surgery, head trauma, epilepsy, central nervous system infections, loss of consciousness, and hydrocephalus. Children who were on medications, which interfered with normal neuropsychological functioning were also excluded from this study.
Sample size
Using the statistical software G*POWER, the sample size was estimated. The input criteria were modeled around the study titled “Prolonged P300 latency in Thyroid failure: A paradox. P 300 latency recovers later in mild hypothyroidism than in severe hypothyroidism” by Tütüncü et al.[17]
The total sample size thus calculated was around 50 for the entire study. However, considering 10 children to be lost to follow-up, we decided to enroll 60 children with hypothyroidism. We lost 8 children to follow-up and ultimately, a total of 52 children were involved in the study.
Diagnosis of hypothyroidism
Diagnosis of hypothyroidism was based on clinical findings and thyroid function tests. Children with either normal or reduced free triiodothyronine (T3) and or free thyroxine (T4) along with increased thyroid-stimulating hormone (TSH) levels (>5 mIU/L) were considered hypothyroid.[1]
Neurophysiological recordings
Nihon Kohden Neuropack X1 was used for recording the latencies and amplitudes of iCNV and lCNV.
CNV was recorded using an oddball paradigm. The recordings were done thrice in each child first at the time of diagnosis, next at 1 month, and finally, at 6-month post-diagnosis. The recording was done under identical conditions each time. The international 10–20 convention was followed for the placement of electrodes.
The following electrodes were placed on the scalp for CNV, frontal (Fz), vertex (Cz), and parietal (Pz) which served as the active electrodes. The forehead (FPz) electrode acted as the ground. A1 and A2 electrodes over the bilateral earlobes acted as the reference electrodes.
The contact impedance of all the surface electrodes was maintained below 5kΩs.
To prevent any confounding in the neurophysiological recordings, the children were advised to have a good sleep the night before the CNV recording. In addition, on the day of recording, they were advised to have a light breakfast and were instructed not to consume any tea, coffee, chocolates, candies, ice creams, wafers, caffeinated or carbonated or sugary drinks before coming for the procedure.
A summary of the methodology has been presented as a flowchart in Figure 1.
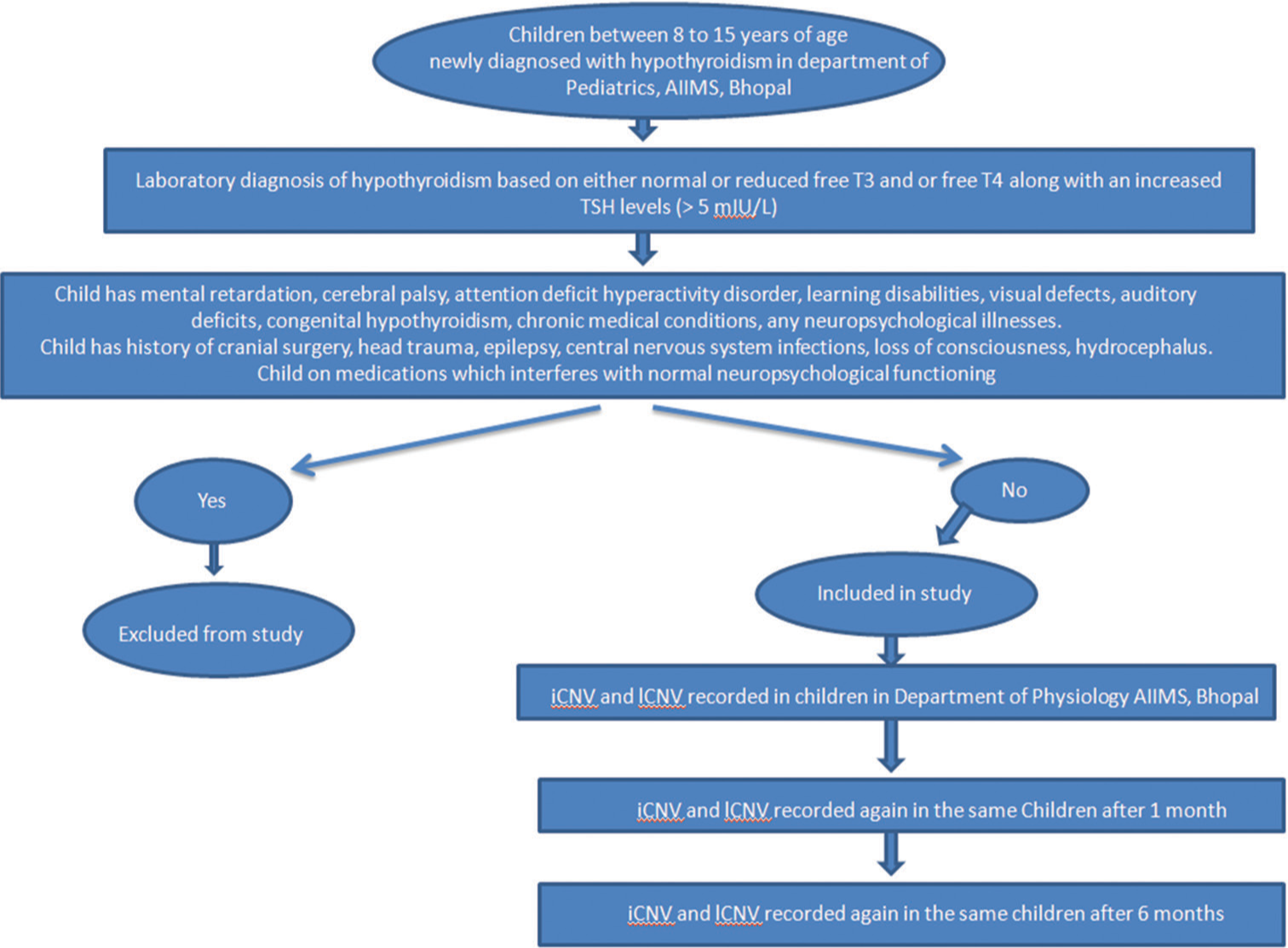
- Flowchart of methodology. iCNV: Initial contingent negative variation, lCNV: Late contingent negative variation.
CNV
These are slow negative brain waves that are detected on EEG recordings when an individual is exposed to two consecutive stimuli that are contingent on one another. The first stimulus is called the warning stimulus and is commonly designated as S1 and the second stimulus is called the imperative stimulus and is designated as S2. Following S2, there is a requirement for a motor action, typically the pressing of the button. CNV is picked up in the interval between these two stimuli, the inter-stimulus interval. The S1 and S2 stimuli can either be auditory or visual or a combination of both.[18]
In this study, both S1 and S2 were auditory in nature. S1 was a single auditory click of 1000 Hz frequency and 100 ms duration. S2 was an auditory burst of 2500 Hz frequency. Immediately after S2, a motor activity in the form of pressing a button was required to terminate the stimulus, and the child was instructed to do the same. The interval between S1 and S2 (inter-stimulus interval) was constant at 3 s throughout the study duration and on follow-ups. The inter-trial interval – the interval between successive trial sets was randomized between 6 s and 10 s. A total of 30 such trials were presented in each session. Although CNV recordings can be fully developed in as few as 5–8 trials, maximum CNV amplitudes are recorded at around 30 trials.[18]
The waves were obtained from the midline Cz, Fz, and Pz with reference electrodes on both earlobes. Each epoch lasted 5000 ms, starting 1000 ms before S1 and ending 1000 ms after S2 with 3000 ms inter S1–S2 interval. A total of 30 trials were performed and they were grand averaged. The period from the onset of recording to the beginning of S1 was taken as a baseline. iCNV amplitudes were taken as the highest amplitude in the interval between 800 and 1100 ms after the onset of S1. lCNV amplitudes were taken as the highest amplitude between 2700 and 3000 ms intervals after the onset of S1 [Figure 2]. The latencies and amplitudes were recorded for analysis.
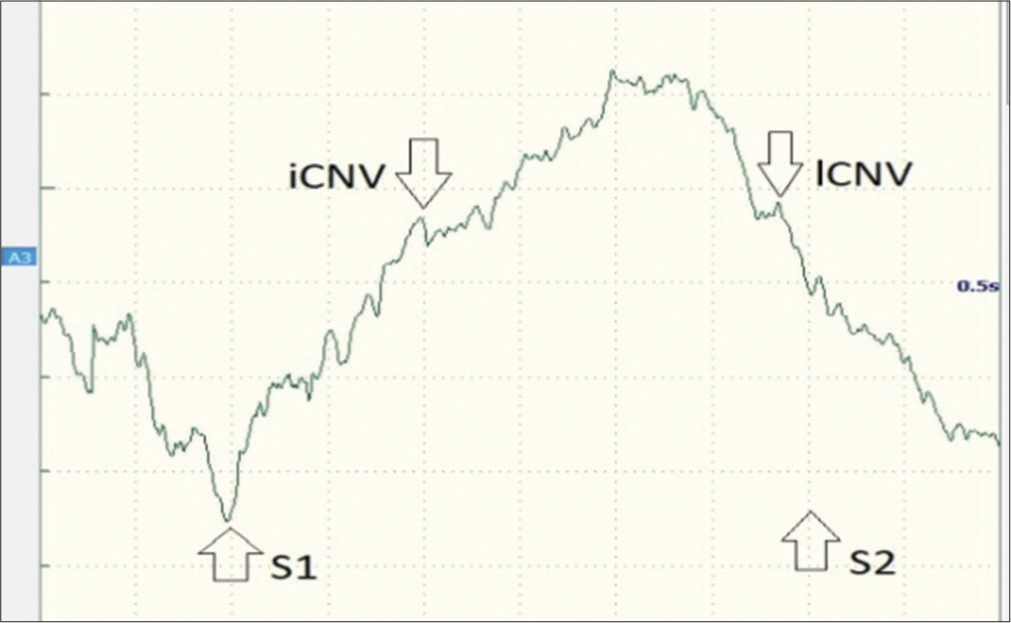
- CNV waveforms that were recorded in a study participant showing iCNV, lCNV, and the onset of S1 (warning stimulus; auditory click) and S2 (imperative stimulus; auditory burst). CNV: Contingent negative variation, iCNV: Initial contingent negative variation, lCNV: Late contingent negative variation.
Statistical analysis
The data were collected and entered into an Excel sheet and checked for any missing values, redundancies, and outliers. Statistical analysis was done using R statistics software version 4.0.0. Shapiro–Wilk test was used to check for the normality of the distribution of data. Variables have been expressed as mean ± standard deviation and categorical data as absolute and percentage values (%). For comparing groups, the Kruskal–Wallis test was applied. A P < 0.05 was considered to be statistically significant.
RESULTS
In this study, a total of 52 children who were newly diagnosed with hypothyroidism were enrolled. The participants included 40 (77%) girls and 12 (23%) boys. The male-to-female ratio was 1:3.33, with a female preponderance.
Demographics
Children ranged in age from 8.3 to 14.9 years. The mean and SD for age was 11.4 ± 1.85 years. The median for age distribution was also 11.4 years. Maximum number of children were in the 9–10-year age group.
Amplitudes of iCNV and lCNV
The iCNV and lCNV amplitudes recorded at the time of diagnosis (baseline) from Fz, Cz, and Pz sites were compared with those obtained during the two follow-ups at the intervals of one month and six months. The values were analyzed for statistical significance using the Kruskal–Wallis test. The changes in amplitudes were not statistically significant [Table 1 and Figure 3].
| iCNV amplitudes (µV) (n=52) | ||||
|
Baseline Mean (±SD) (Median) |
1stFollow-up Mean (±SD) (Median) |
2ndFollow-up Mean (±SD) (Median) |
P-value | |
| Fz | 8.17 (±1.67) (8.15) |
7.42 (±1.77) (7.15) |
8.09 (±1.59) (8.05) |
0.055 |
| Cz | 7.34 (±1.53) (7.30) |
7.54 (±1.33) (7.45) |
7.45 (±1.52) (7.40) |
0.785 |
| Pz | 7.33 (±1.54) (7.30) |
7.53 (±1.34) (7.45) |
7.45 (±1.53) (7.40) |
0.766 |
| lCNV amplitudes (µV) (n=52) | ||||
|
Baseline Mean (±SD) (Median) |
1stFollow-up Mean (±SD) (Median) |
2ndFollow-up Mean (±SD) (Median) |
P-value | |
| Fz | 8.23 (±1.67) (8.20) |
7.92 (±1.78) (8.05) |
7.80 (±1.79) (7.75) |
0.407 |
| Cz | 7.54 (±1.34) (7.60) |
7.36 (±1.53) (7.35) |
7.78 (±1.24) (7.90) |
0.305 |
| Pz | 7.53 (±1.34) (7.50) |
7.34 (±1.52) (7.35) |
7.78 (±1.24) (7.90) |
0.284 |
iCNV: Initial contingent negative variation, lCNV: Late contingent negative variation, Fz: Frontal, Cz: Vertex, Pz: Parietal, SD: Standard deviation
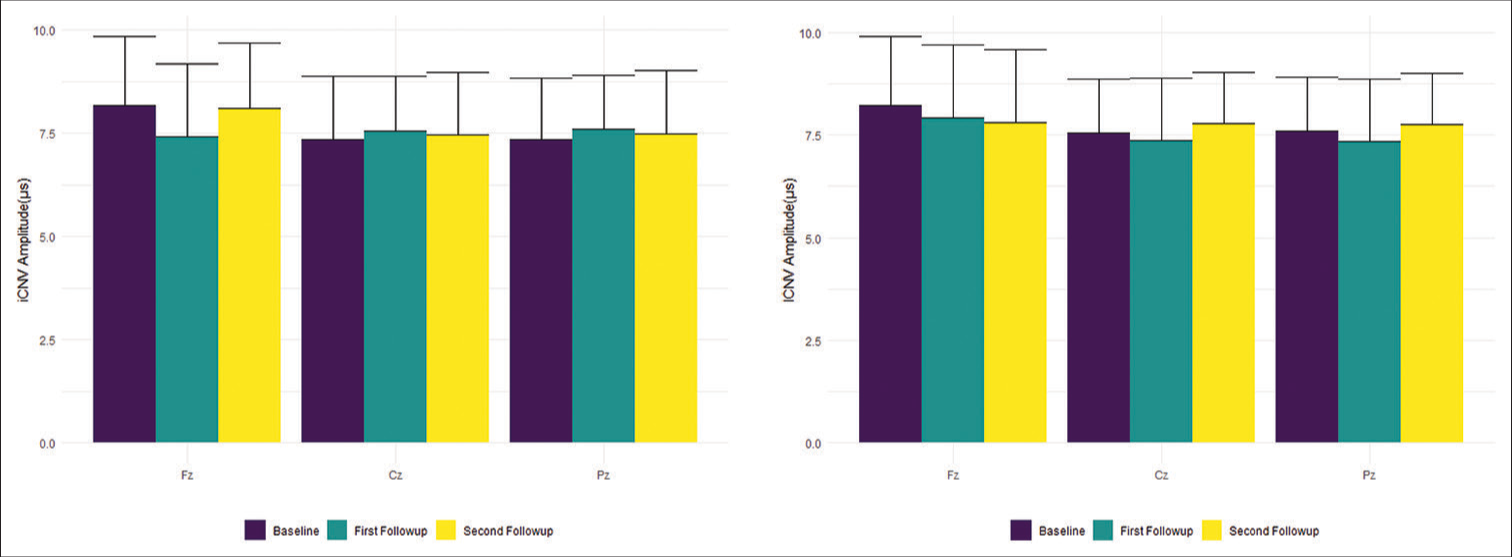
- iCNV (left) and lCNV (right) amplitudes in mV (Mean ± Standard Deviation) at various sites. iCNV: Initial contingent negative variation, lCNV: Late contingent negative variation.
Latencies of iCNV and lCNV
The latencies of iCNV and lCNV (in ms) were measured at Fz, Cz, and Pz sites in each of the participants at baseline, 1st, and 2nd follow-up. Upon applying the Kruskal–Wallis test, the changes in the latencies at the different sites did not show any statistical significance [Table 2 and Figure 4].
| iCNV latencies (ms) (n=52) | ||||
|
Baseline Mean (±SD) (Median) |
1stFollow-up Mean (±SD) (Median) |
2ndFollow-up Mean (±SD) (Median) |
P-value | |
| Fz | 942 (± 80) (947) |
948 (± 88) (942) |
976 (± 82) (981) |
0.096 |
| Cz | 965 (± 83) (964) |
945 (± 81) (933) |
958 (± 81) (968) |
0.384 |
| Pz | 965 (± 83) (964) |
944 (± 80) (934) |
958 (± 82) (967) |
0.385 |
| lCNV latencies (ms) (n=52) | ||||
|
Baseline Mean (±SD) (Median) |
1stFollow-up Mean (±SD) (Median) |
2ndFollow-up Mean (±SD) (Median) |
P-value | |
| Fz | 2,760 (±83) (2,754) |
2,755 (±101) (2,782) |
2,745 (±97) (2,732) |
0.738 |
| Cz | 2,756 (±73) (2,739) |
2,761 (±65) (2,770) |
2,779 (±75) (2,790) |
0.155 |
| Pz | 2,755 (±74) (2,738) |
2,761 (±66) (2,771) |
2,781 (±75) (2,790) |
0.157 |
iCNV: Initial contingent negative variation, lCNV: Late contingent negative variation, Fz: Frontal, Cz: Vertex, Pz: Parietal, SD: Standard deviation
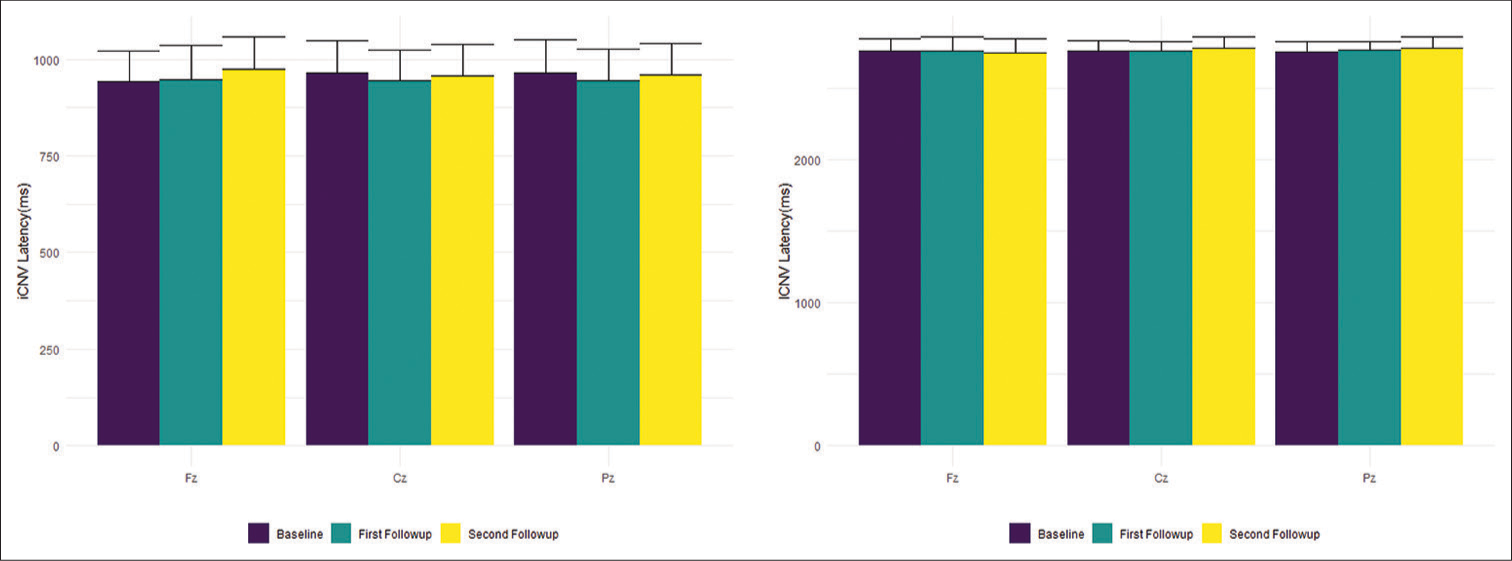
- iCNV (left) and lCNV (right) latencies in ms (Mean ± Standard Deviation) at various sites. iCNV: Initial contingent negative variation, lCNV: Late contingent negative variation.
DISCUSSION
Hypothyroidism is one of the most common pathological hormone deficiency disorders and was described for the very first time in the 19th century. The disease can manifest itself in a wide variety of clinical features, ranging from outright asymptomatic individuals to coma with multiorgan failure in very severe cases.[19]
The thyroid gland synthesizes and releases two major thyroid hormones, namely 3,3’,5-T3 considered the active form, and 3’,5’,3,5-tetraiodo-L-thyronine (T4) which is considered as a prohormone. The majority of the hormone released by the thyroid gland is T4, but, it has 10 times less affinity to nuclear thyroid hormone receptors compared to T3. Once in circulation, the major portion of the thyroid hormones binds to carrier proteins such as albumin, thyroxine-binding globulin, and transthyretin. Only a tiny fraction of the thyroid hormones in circulation is present in the free form. Transthyretin is also a major carrier of protein-binding thyroid hormones in cerebrospinal fluid. In the peripheral tissues, prohormone T4 is converted to the active form T3 and inactive form reverse T3 (rT3) by the enzyme deiodinase. There are three major iodothyronine deiodinase enzymes. Type 1 converts T4 to T3 or rT3 (inactive form). Type 2 converts T4 into T3. Type 3 iodothyronine deiodinase converts T4 and T3 into inactive forms T2 and rT3.[20]
The levels of thyroid hormones as well as thyroid-stimulating hormone in blood can be measured biochemically and are used for the diagnosis of thyroid-related disorders. In general, in a hyperthyroid state, the levels of free T3 and T4 are elevated with reduced levels of TSH. Moreover, in hypothyroid states, the levels of free T3 and free T4 are reduced with increased levels of TSH.[3] For this study, a diagnosis of hypothyroidism was made when the child had either normal or reduced free T3 and or free T4 along with an increased TSH level of >5 mIU/L.
Hypothyroidism is a common clinical condition. It can affect individuals across age groups including children. Hypothyroidism is much more commonly seen in females than males.[3] In this study, a total of 52 children with hypothyroidism were enrolled, which included 40 girls (77%) and 12 boys (23%). Overall, in this study, there was a female preponderance, with a male-to-female ratio of 1:3.33. Several other previous studies have also shown hypothyroidism to be much more common in females than males.[2] The reason as to why females are at a higher risk for thyroid-related disorders is the subject of much research. Studies in rats have demonstrated an estrogen receptor-mediated increase in oxidative stress as a key factor for the increased prevalence of thyroid-related disorders in females.[21] The children ranged in age from 8.3 to 14.9 years, with a mean age of 11.4 years. Maximum number of children was in the 9–10-year age group.
The role of thyroid hormones in the development, maturation, and myelination of the neurons of the central nervous system is well established.[3] Thyroid hormones play a critical role in neuronal migration. The migration of neurons during the development stages is influenced by the presence of laminin. Laminin is an extracellular matrix glycoprotein. The various isoforms of laminin guide neuronal migration. The distribution of laminin is regulated by thyroid hormones.[6] Neurofilaments are intermediate filaments, which are made of proteins. These are present in the cytoplasm of neurons and form an important part of their cytoskeletal structure. Neurofilaments in the axon of neurons provide structural support and help determine the diameter of the axon. Neurofilaments also play a key role in the organization of synapses and influence the release of neurotransmitters. The expression of genes that encode neurofilaments is regulated by thyroid hormones.[5] The development, maturation, and physiological functions of the central nervous system are related to a neurotropin called Brain-derived Neurotrophic Factor (BDNF). Severe hypothyroidism has been linked to reduce messenger RNA and protein expression of genes associated with BDNF in neurons.[22] This apart, in vitro experiments, also suggest that thyroid hormones play a key role in regulating genes in astrocytes that regulate its morphology, proliferation, maturation, and differentiation.[23]
As seen, any deficiency of thyroid hormones can lead to serious adverse effects on neuronal development in fetuses and children. Children with hypothyroidism have been known to develop neurological deficits. These deficits might even develop later on in life despite receiving treatment for hypothyroidism.[3,8] Some of these neurological deficits include impairments in cognition, memory, concentration, attention, intelligence, language, and motor co-ordinations.[2,3,8,9] These impairments can be assessed using neurophysiological tests.[2,3,8,9] CNV is one such neurophysiological test that measures event-related potential (ERP).[11,12] However, to date, there have been no studies pertaining to CNV and hypothyroidism.
CNV can be defined as a slow wave negative potential deflection that can be recorded on EEG which is contingent on the association between two successive stimuli and the individual’s intended response to the second stimulus.[24] This slow negative shift in EEG can be observed in the time interval between the first warning stimulus (S1) and the subsequent imperative stimulus (S2).[24] This two stimulus paradigm consists of two major elements, namely “contingency” and “contiguity.” The former represents the probability of occurrence of one event given the occurrence of another event, whereas the latter represents the temporal association between the two events.[25] Following S2, there is usually a motor activity associated. However, instead of a motor response, various other responses such as tactile discrimination, perceptual discrimination, choice response, mental task, or even suppression of motor response may be incorporated.[25] For this study, the associated motor activity involved was the pressing of a button.
The amplitude of CNV is believed to indicate an anticipatory state, expectancy or motivation or intention of carrying out an act, time estimation for an occurring event, priming, attention, processing of information, decision-making, cognition, orientation, concentration, and focus, readying for a motor response among others.[11,12,24] In this study, both iCNV and lCNV amplitudes as well as latencies were recorded in each child at the time of diagnosis, 1-month, and 6-month post-treatment follow-up period.
It has been noticed that deprivation of sleep can diminish CNV amplitude and in some instances, even abolish it.[26] While on the other hand, the intake of stimulants such as caffeine can augment amplitudes of CNV.[27] To minimize any bias in the CNV recordings, children were advised to get adequate sleep the night before the testing. On the day of the test, they were also asked to refrain from consumption of any chocolates or caffeinated or sugary drinks.
Use of benzodiazepines such as nitrazepam can suppress CNV amplitudes.[27] In this study, children who were on any medications that alter normal neurophysiological function were excluded from the study.
Processes such as attention, expectation, conation, and intention all favor the appearance of CNV,[24] whereas anxiety, distraction, a sense of uncertainty, constraint, or surveillance can attenuate CNV.[24] In this study, neurophysiological tests were carried out in a laboratory under artificially controlled conditions. The room was dimly lit, sound insulated, and temperature controlled to ensure maximum comfort and to keep distractions to a minimum. The only person allowed in the room at the time of recording was the child’s parent or guardian, who was seated in the corner of the room and was instructed not to interact with the child during the course of the recording. These measures ensure that the readings obtained are with minimum confounding.
Studies suggest that CNV is generated in basal ganglia and dopamine possibly plays an important role in the underlying biochemical mechanism of CNV.[28,29] In fact, high CNV amplitudes have been associated with increased dopaminergic activity and low CNV amplitudes have been associated with reduced dopaminergic activity.[28] Dopaminergic agonists such as amphetamine and methylphenidate have been known to enhance CNV amplitudes,[29] whereas dopaminergic antagonists such as chlorpromazine[30] and flupentixol attenuate CNV amplitudes.[31] Hence, changes in CNV reflect alterations in dopamine levels of the brain, and consequently, CNV may serve as a marker for basal ganglia-oriented dopaminergic activity.[28-31] Children on medications that were known to alter dopamine levels were excluded from the study.
Animal studies have suggested that thyroid hormone derivatives play an important role in the development, maturation, and protection of dopaminergic neurons in the brain.[32] By extension, CNV patterns may be attenuated in hypothyroidism. In this study, the amplitudes of iCNV and lCNV showed a modest increase during successive follow-ups compared to the baseline values obtained at the time of diagnosis. However, the changes were not statistically significant (P > 0.05) [Figure 3 and Table 1].
Brain regions such as the thalamus, putamen, left supramarginal gyrus, anterior and posterior insula, cingulate cortex, cerebellum, somatomotor cortex, parietal cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, orbital pre-frontal cortex, supplementary motor area, pre-motor cortex, and somatomotor cortex have been associated with the generation of CNV.[33] It has been suggested that thalamocortical interactions regulate the amplitude of CNV.[34]
The underlying neural mechanisms for CNV have been explored by a few studies and some theories have been proposed. One such theory suggests that during CNV and other ERPs, a synchronized postsynaptic potential occurs over several thousands of neurons simultaneously. CNV represents a time-based process of preparation, and hence, it is noticed that CNV increases over time, provided that the increase is over a short duration of a few seconds. This has been suggested to be due to the involvement of a large group of neurons that fire simultaneously.[35] Another explanation for the CNV waveform is the depolarization over an extended cortical area of apical dendrites of cortical pyramidal cells from thalamic afferents.[25] A different theory attributes the slow increase in negativity seen on CNV waveforms to the anticipation of an upcoming and relevant event.[33]
As described earlier, CNV is noticed to have two distinct components: The early or iCNV, previously termed as o-wave (orientation wave), and the lCNV, previously termed as e-wave (expectation wave). iCNV is often associated with the process of orientation and attention to S1 and lCNV is associated with the process of motor preparedness related to S2.[34] The early part of CNV which has a peak around 1 s after the onset of S1 is considered to reflect an orientation process. It is related to the physical intensity of S1. This orientation process is often defined as a pre-attentive and automatic change in central activity and peripheral state in response to a sudden novel environmental stimulus.[34] This early part of CNV has been variously named as early CNV, iCNV,[10] o wave (orientation wave),[12] etc. The late part of CNV consists of a readiness potential and stimulus preceding negativity. It generally reflects motor preparation and stimulus anticipation.[36] In this study, both the latencies and amplitudes of iCNV and lCNV were recorded at the time of diagnosis, at 1-month, and 6-month follow-ups [Tables 1 and 2; Figures 3 and 4]. iCNV amplitude was taken as the peak amplitude in the interval between 1800 and 2100 ms from the start of the recording. lCNV amplitude was taken as the peak amplitude in the interval between 3700 and 4000 ms from the beginning of the recording.
Abnormalities in CNV waveforms have been reported in a number of disorders. Lower CNV amplitudes were recorded from patients with multiple sclerosis[12] and post-traumatic stress disorder.[11] Reduced iCNV amplitudes were seen in patients with Huntington’s disease[13] whereas reduced lCNV amplitudes were seen in individuals with chronic alcohol consumption.[10] Diminished amplitudes of both iCNV and lCNV were also recorded in patients with end-stage renal disease[14] and in children with anorexia nervosa.[15] In this study, both the iCNV and lCNV amplitudes, although not statistically significant (P > 0.05), were marginally lower at the time of diagnosis compared to the follow-up periods. Similarly, there appeared to be an unassuming reduction in latencies of iCNV or lCNV during follow-up; however, these changes were not statistically significant either (P > 0.05). On the other hand, patients with migraine have been reported to demonstrate an increase in CNV amplitudes.[16]
Limitations
One important limitation of the study is the lack of similar assessments in age and gender-matched healthy control groups of children. We would suggest sub-classifying the children based on the etiology of hypothyroidism and recording CNV in each subgroup. Some causes of hypothyroidism are relatively easier to treat, leading to euthyroidism much earlier, compared to other causes.
CONCLUSION
In this study, CNV, a neurophysiological ERP was measured in children with hypothyroidism at baseline (at the time of diagnosis), 1st follow-up (1 month), and 2nd follow-up (6 months). Latencies and amplitudes of iCNV and lCNV were recorded. On analysis of CNV, we did not find any statistically significant changes in neurophysiological parameters. This may be attributed to a shorter time period of follow-up of 6 months. There is a possibility that CNV parameters may show more pronounced changes after a prolonged duration of treatment. In addition, some of the children would get easily distracted while performing the task which led to having the CNV re-recorded a few times.
Ethical approval
The research/study approved by the Institutional Review Board at AIIMS, Bhopal, number MD0062, dated April 17, 2019.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This study was awarded the MD/MS thesis grant 2019 by Indian Council of Medical Research (ICMR).
References
- Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-16.
- [CrossRef] [Google Scholar]
- Residual goitre in the postiodization phase: Iodine status, thiocyanate exposure and autoimmunity. Clin Endocrinol (Oxf). 2003;59:672-81.
- [CrossRef] [Google Scholar]
- Thyroid-related neurological disorders and complications in children. Pediatr Neurol. 2015;52:373-82.
- [CrossRef] [Google Scholar]
- Thyroid hormone-regulated brain mitochondrial genes revealed by differential cDNA cloning. J Clin Invest. 1995;96:893-9.
- [CrossRef] [Google Scholar]
- Regulation of neurofilament gene expression by thyroid hormone in the developing rat brain. Neuroreport. 1999;10:2361-5.
- [CrossRef] [Google Scholar]
- Thyroid hormone regulates the expression of laminin in the developing rat cerebellum. Endocrinology. 1999;140:4221-7.
- [CrossRef] [Google Scholar]
- Hypothyroidism changes adenine nucleotide hydrolysis in synaptosomes from hippocampus and cerebral cortex of rats in different phases of development. Int J Dev Neurosci. 2005;23:37-44.
- [CrossRef] [Google Scholar]
- Chapter 48: Thyroid disease and the nervous system. Part II: Neurologic aspects of systemic disease In: Biller J, Ferro JM, eds. Handbook of clinical neurology. Vol 120. Netherlands: Elsevier; 2014. p. :703-35.
- [CrossRef] [Google Scholar]
- Effects of early high-dose levothyroxine treatment on auditory brain event-related potentials at school entry in children with congenital hypothyroidism. Horm Res. 2006;66:240-8.
- [CrossRef] [Google Scholar]
- Abnormal CNV in chronic heavy drinkers. Clin Neurophysiol. 2003;114:2081-95.
- [CrossRef] [Google Scholar]
- Contingent negative variation in acute trauma patients: A prospective exploratory study. Biol Psychol. 2018;138:126-32.
- [CrossRef] [Google Scholar]
- Contingent negative variation is associated with cognitive dysfunction and secondary progressive disease course in multiple sclerosis. J Clin Neurol. 2014;10:296-303.
- [CrossRef] [Google Scholar]
- Abnormalities of the contingent negative variation in Huntington's disease: Correlations with clinical features. J Neurol Sci. 2007;254:84-9.
- [CrossRef] [Google Scholar]
- Contingent negative variation before and after hemodialysis among patients with end-stage renal disease. J Neurol Sci. 2008;267:70-5.
- [CrossRef] [Google Scholar]
- Contingent negative variation in children with anorexia nervosa. Pediatr Int. 1999;41:285-91.
- [CrossRef] [Google Scholar]
- Disease duration of episodic migraine correlates with modified amplitudes and habituation of contingent negative variation. J Neural Transm (Vienna). 2015;122:877-85.
- [CrossRef] [Google Scholar]
- Prolonged P300 latency in thyroid failure: A paradox. P300 latency recovers later in mild hypothyroidism than in severe hypothyroidism. Thyroid. 2004;14:622-7.
- [CrossRef] [Google Scholar]
- Contingent negative variation (CNV) and psychological processes in man. Psychol Bull. 1972;77:73-108.
- [CrossRef] [Google Scholar]
- Neurological complications of hypothyroidism, hypothyroidism - influences and treatments London: InTech; 2012.
- [Google Scholar]
- Transport, metabolism, and function of thyroid hormones in the developing mammalian brain. Front Endocrinol (Lausanne). 2019;10:209.
- [CrossRef] [Google Scholar]
- Critical period regulation by thyroid hormones: Potential mechanisms and sex-specific aspects. Front Mol Neurosci. 2019;12:77.
- [CrossRef] [Google Scholar]
- Developmental thyroid hormone insufficiency reduces expression of brain-derived neurotrophic factor (BDNF) in adults but not in neonates. Neurotoxicol Teratol. 2011;33:464-72.
- [CrossRef] [Google Scholar]
- Effect of thyroid hormone depletion on cultured murine cerebral cortex astrocytes. Neurosci Lett. 2009;467:58-62.
- [CrossRef] [Google Scholar]
- Task related changes in contingent negative variation (CNV) response of endogenous evoked potentials. Indian J Physiol Pharmacol. 2000;44:311-6.
- [Google Scholar]
- Slow potentials of the cerebral cortex and behavior. Physiol Rev. 1990;70:1-41.
- [CrossRef] [Google Scholar]
- Modification of surface negative slow potential (CNV) in the human brain after total sleep loss. Electroencephalogr Clin Neurophysiol. 1971;30:17-22.
- [CrossRef] [Google Scholar]
- The effect of caffeine, nitrazepam and cigarette smoking on the contingent negative variation in man. Electroencephalogr Clin Neurophysiol. 1974;37:59-71.
- [CrossRef] [Google Scholar]
- Dopamine and CNV: Studies of drugs, disease and nutrition. Electroencephalogr Clin Neurophysiol Suppl. 1991;42:153-64.
- [Google Scholar]
- Stimulant drugs and ERPs. Electroencephalogr Clin Neurophysiol Suppl. 1991;42:135-41.
- [Google Scholar]
- Chlorpromazine effects on brain activity (contingent negative variation) and reaction time in normal women. Psychopharmacologia. 1975;43:293-5.
- [CrossRef] [Google Scholar]
- Delineation of pharmacopsychological effects by means of endogenous event-related brain potentials: An exemplification with flupentixol. Neuropsychobiology. 1985;13:81-92.
- [CrossRef] [Google Scholar]
- Dopamine neuron induction and the neuroprotective effects of thyroid hormone derivatives. Sci Rep. 2019;9:13659.
- [CrossRef] [Google Scholar]
- Contingent negative variation and its relation to time estimation: A theoretical evaluation. Front Integr Neurosci. 2011;5:91.
- [CrossRef] [Google Scholar]
- Brain activity relating to the contingent negative variation: An fMRI investigation. Neuroimage. 2004;21:1232-41.
- [CrossRef] [Google Scholar]
- Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Brain Res Cogn Brain Res. 2004;21:139-70.
- [CrossRef] [Google Scholar]







