Translate this page into:
Complications in mechanically ventilated patients of Guillain–Barre syndrome and their prognostic value
Address for correspondence: Dr. Archana Becket Netto, FF-1, Alpine Court Apartments, 7th B Main, Jakkasandra Block, Koramangala 3rd Block, Bengaluru - 560 034, Karnataka, India. E-mail: archananetto@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The spectrum of various complications in critically ill Guillain–Barre syndrome (GBS) and its effect on the prognosis is lacking in literature. This study aimed at enumerating the complications in such a cohort and their significance in the prognosis and mortality.
Materials and Methods:
Retrospective case record analysis of all consecutive mechanically ventilated patients of GBS in neurology Intensive Care Unit (ICU) of a tertiary care institute for 10 years was done. Demographic, laboratory, and treatment details and outcome parameters were recorded.
Results:
Among the 173 patients were 118 men and 55 women (2.1:1), aged 1–84 years. The average number of ICU complications per patient was 6.8 ± 1.8 (median = 7, range = 1–12). The most common complication was tracheobronchitis (128). Other pulmonary complications were found in 36 patients. The next was metabolic hyponatremia (115) hypokalemia (67), hypocalcemia (13), stress hyperglycemia (10), hyperkalemia (8), hypernatremia (9). Sepsis (40), UTI (47), dysautonomia (27), hypoalbuminemia (76), anemia (75), seizures (8), paralytic ileus (5), bleeding (4), anoxic encephalopathy (3), organ failures (12), deep vein thrombosis (7), and drug rashes (1) were also noted. The complications, considered significant in causing death, Hughes scale ≤ 3 at discharge, prolonged mechanical ventilation (>21 days) and hospitalization (>36 days) were pneumonia, hyponatremia, hypokalemia, urinary infection, tracheobronchial infections, hypoalbuminemia, sepsis, anemia dysautonomia.
Conclusion:
Active monitoring and appropriate and early intervention by the clinician will improve the quality of life of these patients and reduce the cost of prolonged mechanical ventilation and ICU stay.
Keywords
Complications
Guillain–Barre syndrome
Hughes scale
Intensive Care Unit
mechanical ventilation
Introduction
Complications in patients with Guillain–Barre syndrome (GBS) in Intensive Care Unit (ICU) have been described in association with mechanical ventilation, prolonged immobilization, or specific treatments. The outcome in patients with severe GBS including those requiring mechanical ventilation is generally good if they survive the medical complications.[12]
After admission to the ICU, the most serious complications result from mechanical ventilation, circulatory disturbances, thrombosis, starvation, and sepsis.[3] The complications include lower respiratory tract infections, hyponatremia, urinary tract infection, dysautonomia, urinary tract infection, confusion, and cognitive disturbances. Other less frequent complications are tracheostomy site infection, pseudomembranous colitis, deep vein thrombosis (DVT), and heart block requiring cardiac pacemaker, myocardial infarction, pneumothorax cerebrovascular accidents diabetes mellitus hypocalcemia, herpes zoster and hematemesis, bleeding from tracheostomy site and abnormalities of liver and renal functions.[45]
The prevalence of any complication in a GBS patient during their ICU stay has been 82%, with pneumonia and tracheobronchitis being the most common.[6] There have been attempts to predict the recovery in mechanically ventilated patients, using demographic, disease-related, and laboratory and treatment-related factors.[27] Complications were related to long-term functional recovery and time to regain independent ambulation.[8] However, the spectrum of various ICU-related complications in this cohort and how they affect the ICU stay, functional status and mortality is lacking in the literature. Hence, this study aimed at enumerating the various ICU-related complications in mechanically ventilated patients of GBS and the interplay of these factors in the prognosis and mortality.
Materials and Methods
Retrospective case record analysis of all consecutive mechanically ventilated patients of GBS in the neurology ICU of a tertiary care institute during a period of 10 years was done after obtaining approval from the Institute Ethics Committee. The diagnosis of GBS was restricted to those patients satisfying the NINCDS criteria[9] and in whom secondary causes could reasonably be excluded. These patients who presented themselves to the emergency department of the institute were initially evaluated by the neurology team and admitted to the wards as per the availability of beds and closely monitored for any impending respiratory failure or autonomic dysfunction. At the earliest sign of respiratory compromise, the patients were evaluated by the neuroanesthesia team of the institute, and shifted for mechanical ventilation. Rarely patients had to be referred to outside centers for ventilator support due to nonavailability of ICU bed. The criteria for selection of mechanical ventilation were features of hypoxia, vital capacity <15 ml/kg, PaO2 of <70 mmHg, and PaCO2 of >45 mmHg.[5] Those patients who were kept in ICU for only observation and monitoring, not satisfying criteria for GBS and incomplete case records were excluded from this study.
Records were retrieved from the medical records section of the institute and clinical, and laboratory parameters entered in a predesigned Performa. Details of demographic data, antecedent events, progression of symptoms (sensory, bulbar, and respiratory involvement), laboratory parameters and investigations done to exclude the secondary causes, ICU complications, electrophysiological studies, treatment details, and the various outcome parameters in terms of rapidity of progression to nadir, duration of ventilation/ICU stay, time for onset of recovery, duration of hospital stay, day of ambulation, Hughes scale at discharge, and day of death were recorded.
Definition of terms
-
Autonomic dysfunction. Parameters noted include cardiac arrhythmias, labile blood pressure, abnormal hemodynamic response to drugs, electrocardiographic abnormalities, pupillary dysfunction, sweating abnormalities, urinary retention, and gastrointestinal (GI) dysfunction[7]
-
Complications in ICU: Metabolic - electrolyte abnormalities; hyponatremia: Sodium <135 mEq/L, hypernatremia: Sodium >145 mEq/L, hypokalemia: Potassium (K) <3.5 mEq/L, hyperkalemia: K >5.5 mEq/L, hypocalcemia: Calcium <8.5 mg/dl (at least on 2 occasions), organ failures. Nutritional: Hypoalbuminemia = albumin <3.5 g%, anemia = Hb <12 g% (at least on 2 occasions). Pulmonary complication - pneumonia, collapse, pneumothorax as evidenced by an abnormality on chest X-ray sepsis: Fever, tachycardia, increased leukocyte count, bacteremia (blood culture), or central access device contamination as defined by positive pathogenic microbial culture (any 2 or more of the above features in combination). Tracheobronchitis: Pathogenic microbial culture of tracheal or bronchoscopy aspirate. Apart from these, complications such as DVT, cardio/cerebrovascular accidents, bleeding manifestations, and allergic reactions were separately recorded as “Others”
-
The outcome parameters analyzed included the following: (1) In-hospital mortality; (2) GBS disability scale score (Hughes scale: A scale of 0–6 of progressive disability ventilator dependence and death, a score 3 or below indicates that patient can walk 5 m).[7] Day of death: Computed from the onset of illness. Duration of mechanical ventilation: From the day of connecting to ventilator to the day of disconnecting from the ventilator. Duration of ICU stay: Calculated from entry to ICU to exit from ICU. Duration of hospital stay: Taken from the day of presentation to the hospital to day of discharge.
Statistical analysis
Statistical analysis was performed using SPSS Inc. SPSS for Windows, Version 14.0. Chicago, SPSS Inc. Univariate analysis was done using analysis of variance for continuous variables and Chi-square test for categorical variables. Chi-square test or Fischer's exact test was done to compare two groups. The variables, which emerged as significant in univariate analysis, were used for multivariate analysis using logistic regression analysis to find out which variables were helpful in predicting the outcomes.
Results
Description of the cohort
Over a period of 10 years, 191 patients of GBS were admitted to the ICU of this major tertiary care neurological center in India. Of this 13 patients had been kept in ICU for only observation and five case records lacked adequate data for the study and hence were excluded.
Among the 173 patients, 118 were men and 55 were women (2.1:1), the age varied from minimum of 1 year to maximum of 84 years. History of a prior antecedent event was given by 48% of the patients and included fever - 68, GI illness - 16, respiratory infection - 13 vaccination - 2, surgery – 1, and 14 others which included specific infections.
Eighteen patients died. Three were ventilator dependent at the time of discharge of which one patient was in a vegetative state but had been discharged against advice. Relatives took the other two patients to a center of their choice. The Hughes scale at discharge could not be elucidated from the case record in three patients. Forty-seven patients were ambulant with or without support at the time of discharge. The day of death ranged from 5 days to 196 days (39 ± 40.3 days). The median duration of ventilation and duration of hospitalization was 20.5 and 36 days respectively and hence prolonged ventilation and hospitalization were categorized as >21 days and >36 days. The mean was not chosen as the standard deviation varied widely (29.9 ± 26.3 and 50.5 ± 41.2 days).
Complications during stay in Intensive Care Unit
It was found that the average number of ICU complications per patient was 6.8 ± 1.8 (median = 7, range = 1–12). The most common complication was tracheobronchitis (128), which was considered whenever culture from the tracheobronchial tree was positive [Table 1]. Pulmonary complications other than tracheobronchitis were found in 36 patients (pneumonia - 21, collapse - 9, effusion - 3, pneumothorax - 3). Two of the patients with pneumothorax one patient with pleural effusion and two patients with collapse had obtained an opinion from chest physician and had undergone procedures of intercostal drainage tube insertion, and bronchoscopic removal of the mucus plug, respectively. The next common complication was metabolic, especially hyponatremia (115) followed by hypokalemia (67), hypocalcemia (13), hyperkalemia (8), hypernatremia (9). Sepsis was found in 40, UTI in 47, dysautonomia in 27, hypoalbuminemia in 76, anemia in 75, seizures in 8, paralytic ileus in 5, and bleeding in 4. All the four patients who had cardiac dysfunction had been evaluated by a cardiologist with electrocardiography ECG, echocardiography, and cardiac enzymes and treated accordingly. Four patients had elevated urea of more than 40 and creatinine of more than 1.5 and had been seen by nephrologist and managed conservatively with avoidance of nephrotoxic drugs and fluid and electrolyte corrections and diuretics. The four patients with liver dysfunction had an abnormal elevation of liver enzymes and bilirubin. Of this, one patient had a history of congenital hyperbilirubinemia and another patient's liver enzymes were elevated and were clinically diagnosed to have viral hepatitis. This patient's hepatitis B surface antigen and HCV antibody titers were negative. Anoxic encephalopathy was attributed to prolonged Ambu ventilation and cardiac arrest while on ventilator. The duration of Ambu ventilation before shifting to ICU in these three patients ranged from 1 day to 7 days and at the time of discharge two patients were in a chair bound state and one patient's Hughes scale could not be computed from the case record. Among the patients who sustained cardiac arrest two died subsequently and one was discharged in a persistent vegetative state dependent on ventilator. The ten patients who had hyperglycemia requiring intervention in the ICU did not have the previous history of diabetes, and hence this could be stressed induced hyperglycemia or subclinical diabetes mellitus detected for the first time. Rashes seen in one patient were interpreted in the records as cephalosporin-induced and had disappeared after stopping cephalosporins. All the seven patients who had DVT had swelling of the leg, had undergone Doppler studies, and had proven thrombophlebitis. One patient had bed sore.

The complications that were considered significant in causing death, Hughes scale ≤3, prolonged mechanical ventilation for more than 21 days or prolonged hospitalization more than 36 days were pneumonia, hyponatremia, hypokalemia, urinary infection, tracheobronchial infections, hypoalbuminemia, sepsis, anemia dysautonomia [Table 2].
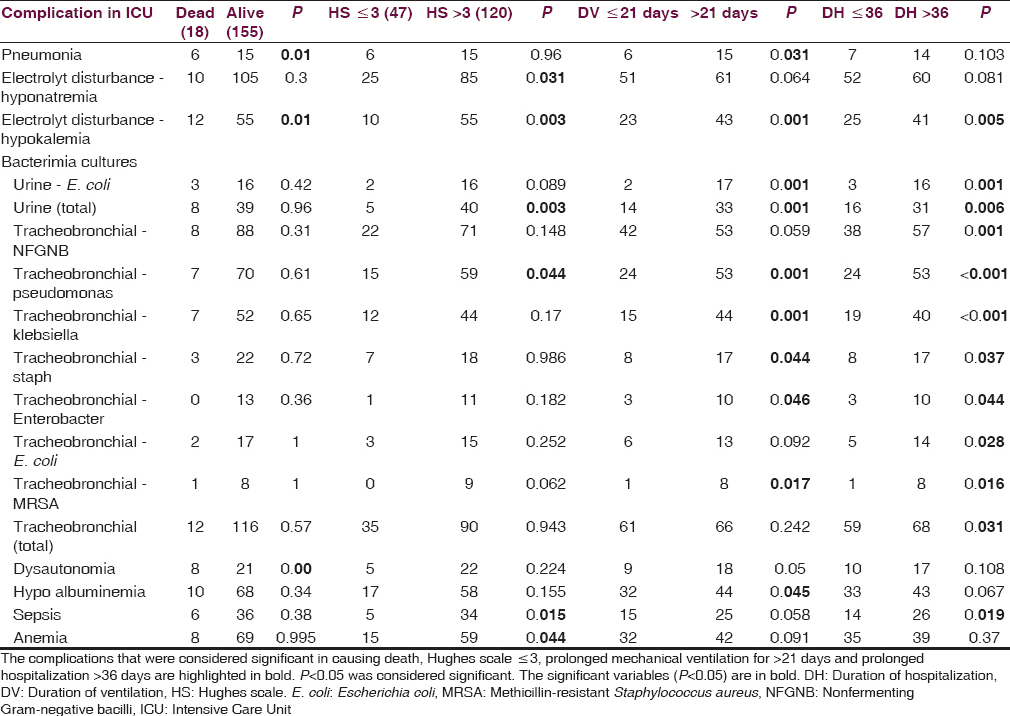
The various organisms isolated were Escherichia coli, Klebsiella, Enterococcus from urine and nonfermenting Gram-negative bacilli, Pseudomonas aeruginosa, Klebsiella, E. coli, Staphylococcus aureus, Enterobacter, methicillin-resistant S. aureus from endotracheal secretions.
The incidence of anemia and hypoalbuminemia was significantly more in the plasmapheresis (small volume and large volume) group. Large volume plasmapheresis (LVP) made an impact in reducing the mortality. There was no significant difference in terms of lung collapse, hypocalcemia, hypernatremia, hyperglycemia needing treatment (organ failures especially renal and cardiac), or treatment received in the form of steroids, small volume plasmapheresis, intravenous immunoglobulin, or combination of any of these treatment modalities.
Binary Logistic Regression analysis of the significant variables
Binary Logistic Regression analysis was used to predict the condition at discharge (dead vs. alive) using the variables significant in the univariate analysis. Age, complication in ICU – pneumonia, electrolytic disturbances – hypokalemia, dysautonomia, large volume plasma exchange treatment were used as independent variables (predictors) and condition at discharge (mortality) as the dependent variable [Table 3].
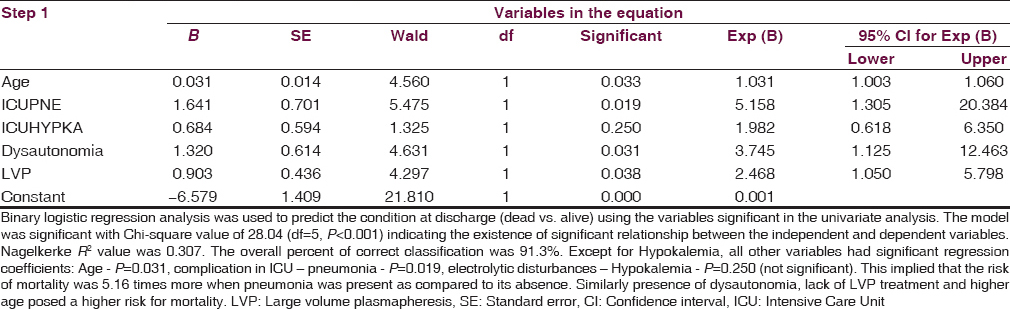
The model was significant with Chi-square value of 28.04 (df = 5, P < 0.001) indicating the existence of a significant relationship between the independent and dependent variables. Nagelkerke R2 value was 0.307. The overall percent of correct classification was 91.3%. Except for hypokalemia, all other variables had significant regression coefficients - age P = 0.031, complication in ICU – pneumonia P = 0.019, electrolytic disturbances – hypokalemia P = 0.250 (not significant), dysautonomia P = 0.031, LVP P = 0.038. The odds ratio was highest for pneumonia (5.158) followed by dysautonomia (3.745), large volume plasma exchange (2.468), and age (1.031). This implied that the risk of mortality was 5.16 times more when pneumonia was present as compared to its absence. Similarly presence of dysautonomia, lack of LVP treatment, and higher age posed a higher risk for mortality.
For other three variables (Days in Hospital, Duration of ventilation and Hughes Scale) only two-way analysis (cross tables with Chi-square or Fisher's test) results are to be used, as multivariate analysis did not show any significant results.
Discussion
The incidence of tracheobronchitis, hyponatremia, and hypokalemia is very high compared to the other studies [Table 2].[10] This could probably be due to the dilutional effects of replacement fluids given following plasmapheresis that was carried out in majority of the patients. The frequency of anemia and hypoalbuminemia was lacking in the other studies; however, in the current study, it was felt that this would reflect the nutritional status of the patients and by affecting the various reparative processes would, in turn, have an impact on the duration of ICU and hospital stay. The results reveal it did affect the functional status at discharge and the duration of mechanical ventilation. The incidence of DVT, stress hyperglycemia, abnormalities of liver function is in agreement with other studies. Even though there are studies that have concentrated exclusively on the complications of ventilated patients of GBS.[610] The present study has the largest number of critically ill patients and analyzed a wider spectrum of complications [Table 2] and assessed the prognostic significance of the same.
Various studies have shown ICU complications lead to slower recovery[8] especially ventilator-associated pneumonia[11] and other pulmonary complications.[12] Pulmonary complications have been correlated with higher mortality.[1314] It is well known that hypokalemia delays weaning from respirator. In our cohort, hypokalemia increased mortality, prolonged duration of ventilation and hospitalization as well as worsened the functional status at discharge. There is recent literature to evaluate the prognostic value of hyponatremia in patients with GBS concluding low sodium levels predicted mortality (Wang 2015) however current study reveals it to be affecting the functional status at discharge as well. Anemia has been previously reported as a frequent complication but one that does not interfere with functional recovery.[15] Knowledge of the various organisms isolated from each ICU helps in tailoring the antibiotics to the specific microbe and reduces the cost as well as the duration of ventilation and hospital stay. Sepsis has been correlated with higher mortality in these patients.[14] We found it to be leading to a worsened Hughes scale at discharge and prolonged hospitalization. The role of neurologist does not end with the prescription of various immunomodulatory treatment as it is evident that many of these complications affect the outcome in terms of mortality, duration of ventilation, ICU, and hospital stay as well as the functional status achieved at the time of discharge. The outcome parameters that are commonly described such as gender, type of antecedent events, onset to peak, bulbar symptoms, severity of weakness, and dysautonomia, are all nonmodifiable whereas all the complications studied and enumerated in the present study are modifiable. Active monitoring and appropriate and early intervention by the clinician will go a long way in improving the quality of life of these patients and reduce the cost of prolonged mechanical ventilation and ICU stay.
Conclusion
The study has the largest cohort of mechanically ventilated patients of GBS and has enumerated the maximum number of ICU complication compared to any other study till now in literature. The wide spectrum of complications may reflect the meticulous documentation of the record and provides an opportunity for prevention of complications. It sensitizes the treating clinician about the various day-to-day problems seen in such patients and to actively look for all these complications. However, the retrospective nature of the study on the cohort over 10 years may have the disadvantage of nonuniformity of protocols followed regarding treatment, electrophysiological studies and discharge policies. During the 10-year studied, there could have been sea changes in equipment and quality of ICU care, personnel, and inconsistent charting. How this could have affected the outcome needs a separate study. The long-term follow-up data were not available for almost 50% of the cohort and hence was not studied. A prospective study of mechanically ventilated patients of GBS may resolve these shortcomings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Long-term outcome in patients with Guillain-Barré syndrome requiring mechanical ventilation. Neurology. 2000;54:2311-5.
- [Google Scholar]
- Intensive management and treatment of severe Guillain-Barré syndrome. Crit Care Med. 1993;21:433-46.
- [Google Scholar]
- The morbidity of Guillain-Barré syndrome admitted to the intensive care unit. Neurology. 2003;60:17-21.
- [Google Scholar]
- Mortality in mechanically ventilated patients of Guillain Barré Syndrome. Ann Indian Acad Neurol. 2011;14:262-6.
- [Google Scholar]
- The morbidity and outcome of patients with Guillain-Barré syndrome admitted to the intensive care unit. J Neurol Sci. 2008;264:121-8.
- [Google Scholar]
- Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(Suppl 1):S21-4.
- [Google Scholar]
- Hyponatremia is a predictor for poor outcome in Guillain-Barré syndrome. Neurol Res. 2015;37:347-51.
- [Google Scholar]
- Mechanical ventilation in patients with Guillain-Barré syndrome. Respir Care. 2006;51:1403-7.
- [Google Scholar]
- Factors predicting extubation success in patients with Guillain-Barré syndrome. Neurocrit Care. 2006;5:230-4.
- [Google Scholar]
- Prognosis of patients with Guillain-Barré syndrome requiring mechanical ventilation. Neurol India. 2011;59:707-11.
- [Google Scholar]
- Guillain-Barré syndrome: Incidence and mortality rates in US hospitals. Neurology. 2008;70:1608-13.
- [Google Scholar]






