Translate this page into:
Clinicoradiological features of cerebral microbleeds diagnosed on magnetic resonance neuroimaging
*Corresponding author: Krishnan Nagarajan, Department of Radio-Diagnosis, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India. lknagarajan1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bharath J, Amuthabharathi M, Sivasubramaniyan KM, Adithan S, Narayan SK, Sunitha VC, et al. Clinicoradiological features of cerebral microbleeds diagnosed on magnetic resonance neuroimaging. J Neurosci Rural Pract. 2024;15:300-6. doi: 10.25259/JNRP_331_2023
Abstract
Objectives:
Cerebral microbleeds (MBs) are recently described entity on magnetic resonance (MR) neuroimaging and are considered one of the markers of small vessel disease. We aimed to study the clinicoradiological features of cerebral MBs that were diagnosed in MR neuroimaging.
Materials and Methods:
We studied 109 South Indian patients, who presented to a tertiary care institution for MR neuroimaging with cerebral MBs as diagnosed on MR neuroimaging based on either the gradient T2* imaging or susceptibility-weighted imaging. The clinical details and coexisting MR features of infarcts, macrohemorrhages, lacunar infarcts, and white matter leukoaraiosis were evaluated and analyzed.
Results:
Of 109 patients, 79 were males and 30 were females. Associated clinical comorbidities noted include hypertension (62.39%), diabetes (23.85%), and alcoholism (31.19%) apart from the history of anti-platelet/anti-coagulant usage (15.5%), previous cardiac disease (12.84%), and previous stroke/transient ischemic attacks (9.17%). Other co-existing neuroimaging abnormalities noted include cortical infarcts (27.52%), old hemorrhages (29.36%), lacunar infarcts (56.88%), and white matter leukaraiosis (67.89%).
Conclusion:
The clinicoradiological features of cerebral MBs in South Indian patients are similar to other Asian and Western studies with significant coexistence of clinical comorbidities and imaging features of small vessel changes. Further studies with a larger sample are needed to correlate the grade of MBs to the individual risk of these clinicoradiological characteristics.
Keywords
Microbleeds
Cerebral microbleeds
Magnetic resonance imaging
Gradient echo T2* imaging
Susceptibility-weighted imaging
INTRODUCTION
Cerebral microbleeds (MBs) are defined as small perivascular hemosiderin deposits, which are small, round or ovoid lesions of the cerebral parenchyma of low signal intensity on T2*-weighted and Gradient echo (GRE) magnetic resonance imaging (MRI) sequences, with a maximal diameter of 5–10 mm.[1,2] Several studies have been carried out in the recent past to identify the risk factors of cerebral MBs. Hypertensive vasculopathy, amyloid angiopathy, renal impairment with hyperuricemia, male gender, old age, smoking, heavy alcohol consumption, hyperlipidemia, diabetes mellitus, previous antithrombotic agents, and atrial fibrillation are among the risk factors recognized as associated with cerebral MBs.[3-5] Assessing the topographical distribution of cerebral MBs provides a diagnostic clue about the underlying disease. For example, both hypertensive small-vessel disease and cerebral amyloid angiopathy (CAA) contribute to the formation of lobar cerebral MBs while cerebral MBs located in the basal ganglia or infratentorial brain regions are mainly associated with hypertensive vasculopathy.[4] With the advancement in MRI technology, there is a steady rise in the identification of cerebral MBs. From the clinical point of view, early detection and treatment of the underlying etiology of MB,s which will definitely improve, leading to better understanding and possible prognostication. Recent studies have shown that cerebral MBs are an independent predictor of stroke outcomes and recurrences.[5] We aimed in this study to describe the clinicoradiological features of MRI-diagnosed cerebral MBs.
MATERIALS AND METHODS
We included 109 patients, who were diagnosed to have cerebral MBs on MRI over 2 years (from January 2018 to December 2019) after approval from the Institute Research and Ethics Committees. All patients diagnosed with MBs in MRI were included and those without clinical details were excluded from the study. Detailed history and clinical details including functional status and comorbidity were obtained from the case files maintained at the medical records department. The demographic characteristics of the study patients, including their vascular risk factors and previous use of antithrombotic agents (antiplatelet agents and anticoagulants), were also collected from medical records. The risk factors considered include hypertension, diabetes mellitus, previous cardiovascular disease, smoking, alcoholism, previous stroke or transient ischemic attacks (TIAs), anti-platelet or anti-coagulant use, history of head injury or neurosurgical procedures, radio- or chemo-therapy, and uremia (with or without dialysis).
MRI was performed with a routine 1.5 T MRI (Avanto Siemens, Erlangen, Germany) and included either GRE MRI sequences (TR/TE 900/26 ms, slice thickness 4 mm, flip angle 20°, matrix 256 × 192, FoV 230 mm, and No. of averages 1) or susceptibility-weighted imaging (SWI) sequence (TR/TE 49/40 ms, flip angle 15 o, matrix size 256 × 200, FoV = 230 × 180 mm, slice thickness 3 mm, and No. of averages 1). Thirty-nine patients had GRE sequence imaging and 70 patients had SWI imaging. About 78 patients had undergone MRI in 1.5T and the remaining 31 in 3T MRI.
Magnetic resonance (MR) brain images were analyzed in detail to detect cerebral MBs and their location. The lesions are divided based on the size and location (1. Lobar CMBs [a. frontal, b. parietal c. temporal, d. occipital] 2. Deep CMBs, 3. Cerebellar CMBs). All these details were entered in the data collection pro forma. Based on the total number of cerebral MBs, patients were classified as (a) <5 cerebral MBs – grade 1, (b) 6–10 cerebral MBs – grade 2, (c) 11–25 cerebral MBs – grade 3, and (d) >25 cerebral MBs – grade 4.[6] On magnetic resonance angiography (MRA), the cerebral vascular variants such as a hypoplastic vertebral artery and fetal posterior cerebral artery (PCA) were also documented. We subgrouped the MB patients into predominantly lobar and predominantly deep cerebral groups based on the topographical distribution of cerebral MBs and explored the association of these two major subgroups with various baseline clinicoradiological characteristics.
Statistical analysis
The data were tabulated and descriptive statistical analysis of the data was done using IBM Statistical Package for the Social Sciences software version 2.0. The association of the radiological profile with categorical variables such as gender and other risk and comorbidity factors, stroke subtypes, and outcome parameters was carried out using the Chi-square test/Fisher exact test. All analyses were done at a 5% level of significance with a P < 0.05 considered as statistically significant.
RESULTS
Out of 109 patients, there were 79 (72.5%) males and 30 (27.5%) females with the predominant age group of 18–65 years (79.8%). There were four patients of <18 years and 18 were older than 65 years. The comorbid factors were as follows; hypertension 62.4%, diabetes 23.8%, alcoholism 31.2%, and smoking 9.2%. The other predisposing factors were as follows: anti-platelet/anti-coagulant use 15.5%, cardiovascular disease including rheumatic valvular disease/atrial fibrillation 12.8%, previous stroke/TIAs 9.2%, history of radio- or chemo-therapy 8.3%, and previous neurosurgery and head injury 4.6% each. Uremia (+ dialysis) was seen in 5.5%, systemic infections (two out of three were hepatitis B) in 2.75%, suspected cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in 5.5%, and vasculitis in one patient [Table 1].
| Clinical characteristics | Number (Percentage) |
|---|---|
| Total patients | 109 |
| Male | 79 (72.48) |
| Female | 30 (27.52) |
| Age group <18 years | 4 (3.67) |
| 18–65 years | 87 (79.82) |
| >65 years | 18 (16.51) |
| Hypertension | 68 (62.39) |
| Diabetes mellitus | 26 (23.85) |
| Alcoholic | 34 (31.19) |
| Smokers | 10 (9.17) |
| Predisposing/risk factors | |
| H/o Head injury | 5 (4.59) |
| H/o Neurosurgery | 5 (4.59) |
| H/o Radio-/Chemo-therapy | 9 (8.26) |
| H/o TIAs/Stroke | 10 (9.17) |
| H/o Cardiovascular disease/Rheumatic heart disease/Atrial fibrillation | 14 (12.84) |
| H/o Antiplatelet/anticoagulant use | 17 (15.6) |
| Other associations | |
| Renal disease – Uremia/AKI | 6 (5.5) |
| Infections | 3 (2.75) |
| CADASIL (possible) | 6 (5.5) |
| Vasculitis | 1 (0.92) |
| Others (Postpartum, etc.) | 21 (19.26) |
AKI: Acute kidney injury, H/o: History of, CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, TIA: Transient ischemic attack.
Imaging findings
Out of 109 patients, deep microbleeds were noted in 90 (82.6%), deep with cerebellar 105 (96.3%), and cortical and lobar in 91 (83.5%). The grades of these patients include; Grade 1 (<5 MBs) in 15 (13.8%) patients, Grade II (6–10 MBs) in 25 (22.9%), Grade III (11–25 MBs) in 36 (33%), and Grade IV (>25 MBs) in 33 (30.3%) patients [Figures 1-5, Table 2]. Cortical or territory infarcts were noted in 30 (27.5%), previous hemorrhages (basal ganglia or lobar) in 32 (29.4%) lacunar infarcts in 62 (56.9%), and white matter leukoaraiosis in 74 (67.9%). We also tried to see MRA which is done routinely for all patients with neurovascular/stroke work-up. Vessel narrowing/occlusions were noted in 7 (6.4%) patients and dolichoectasia (vertebra-basilar) in 26 (23.8%) apart from normal variants (including fetal PCA, hypoplastic vertebral artery + thin vertebra-basilar arteries) in 47 patients [Table 3].
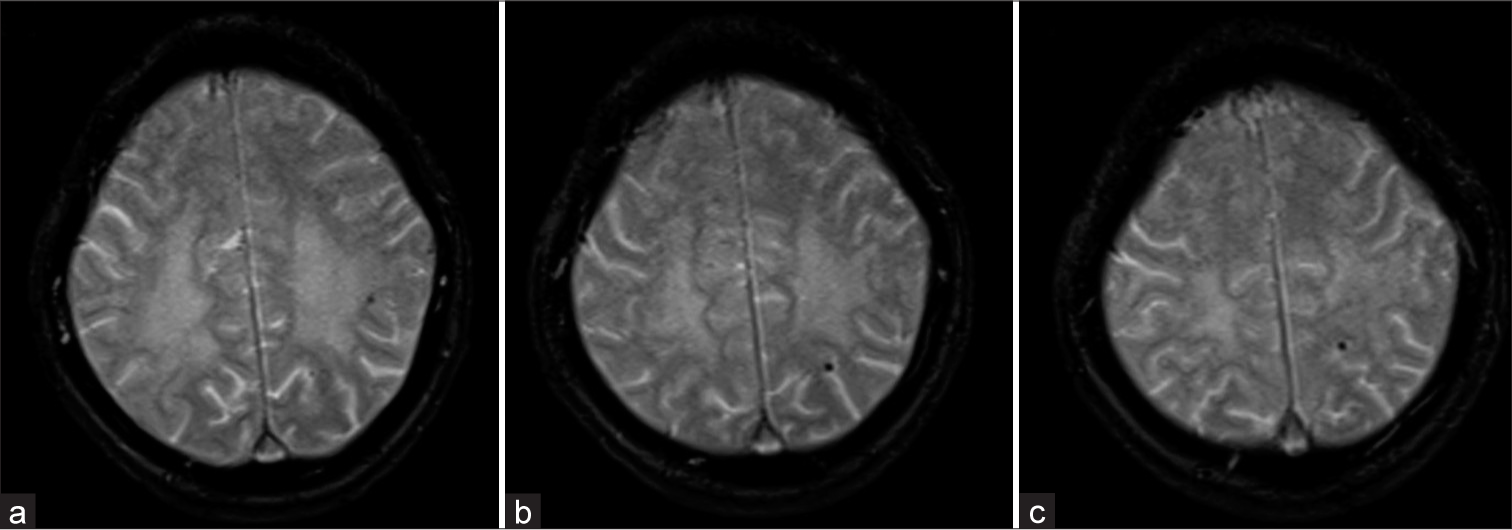
- (a-c) GRE T2* axial images above the level of ventricles showing Grade I (<5) lobar microbleeds.
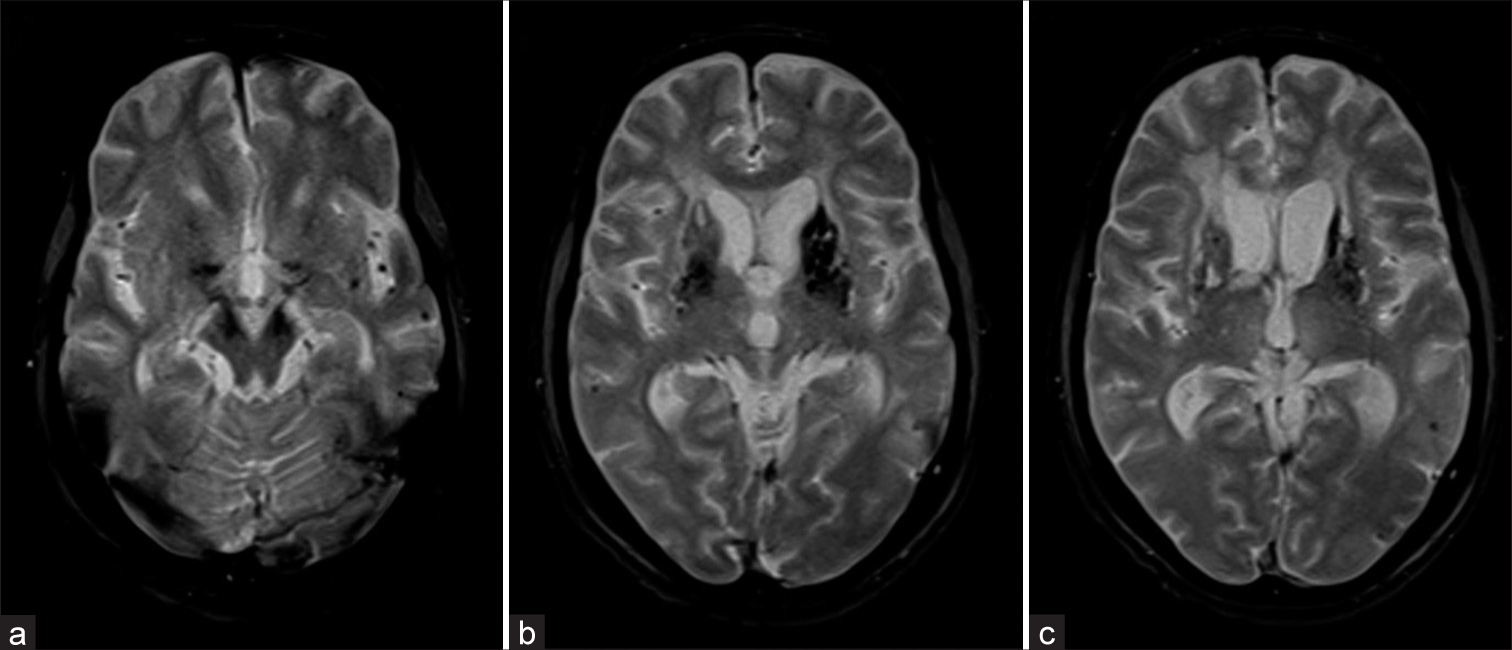
- (a-c) The GRE T2* axial images at posterior fossa and basal ganglia level showing Grade II (6–10) deep and lobar microbleeds with the left putaminal old (macro) hemorrhage.
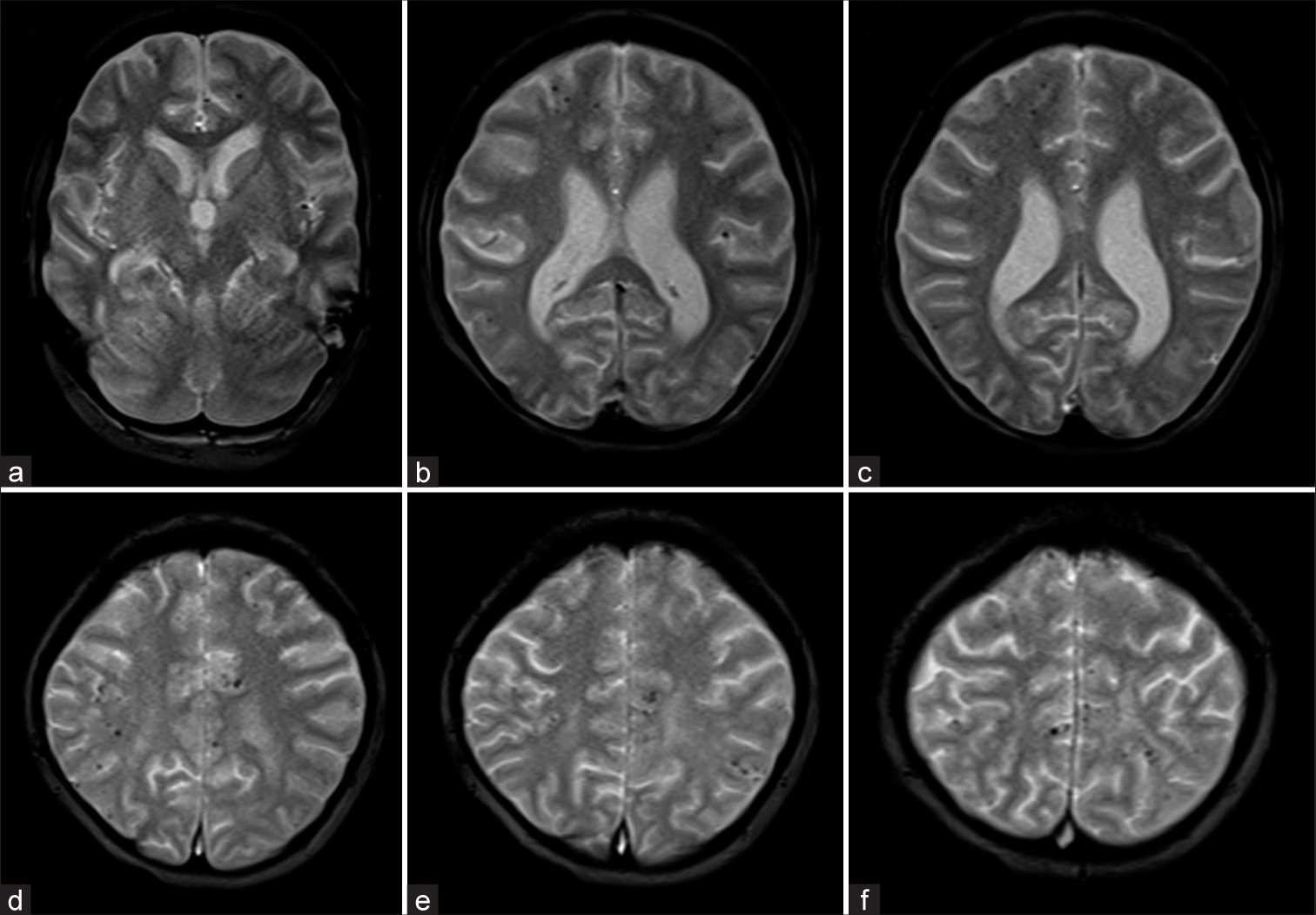
- (a-f) GRE T2* axial images showing Grade III (11–25) microbleeds restricted to only lobar location without deep microbleeds.
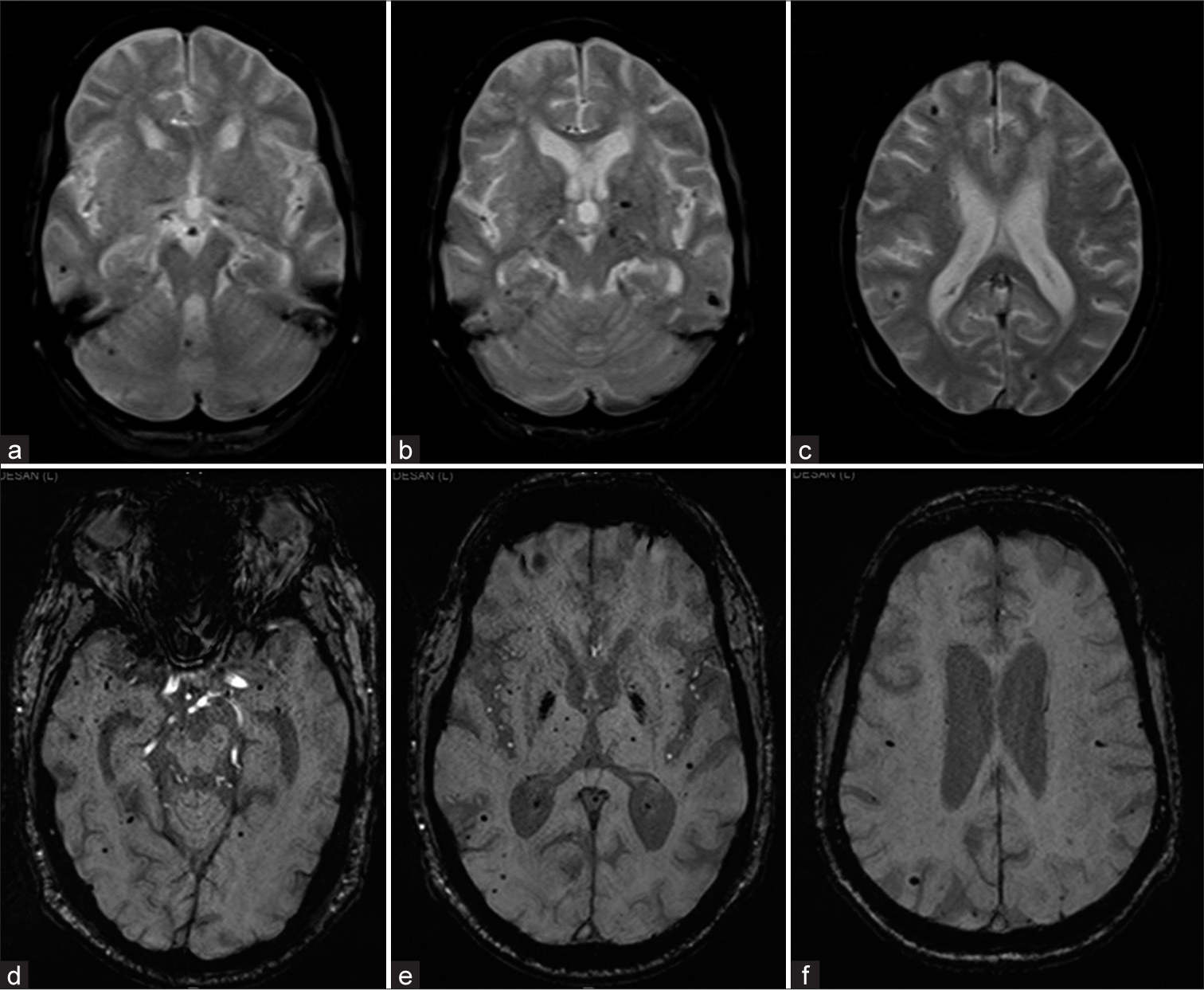
- Two different patients with (a-c) GRE T2* axial and (d-f) susceptibility-weighted imaging axial images showing Grade III microbleeds of deep and lobar location.
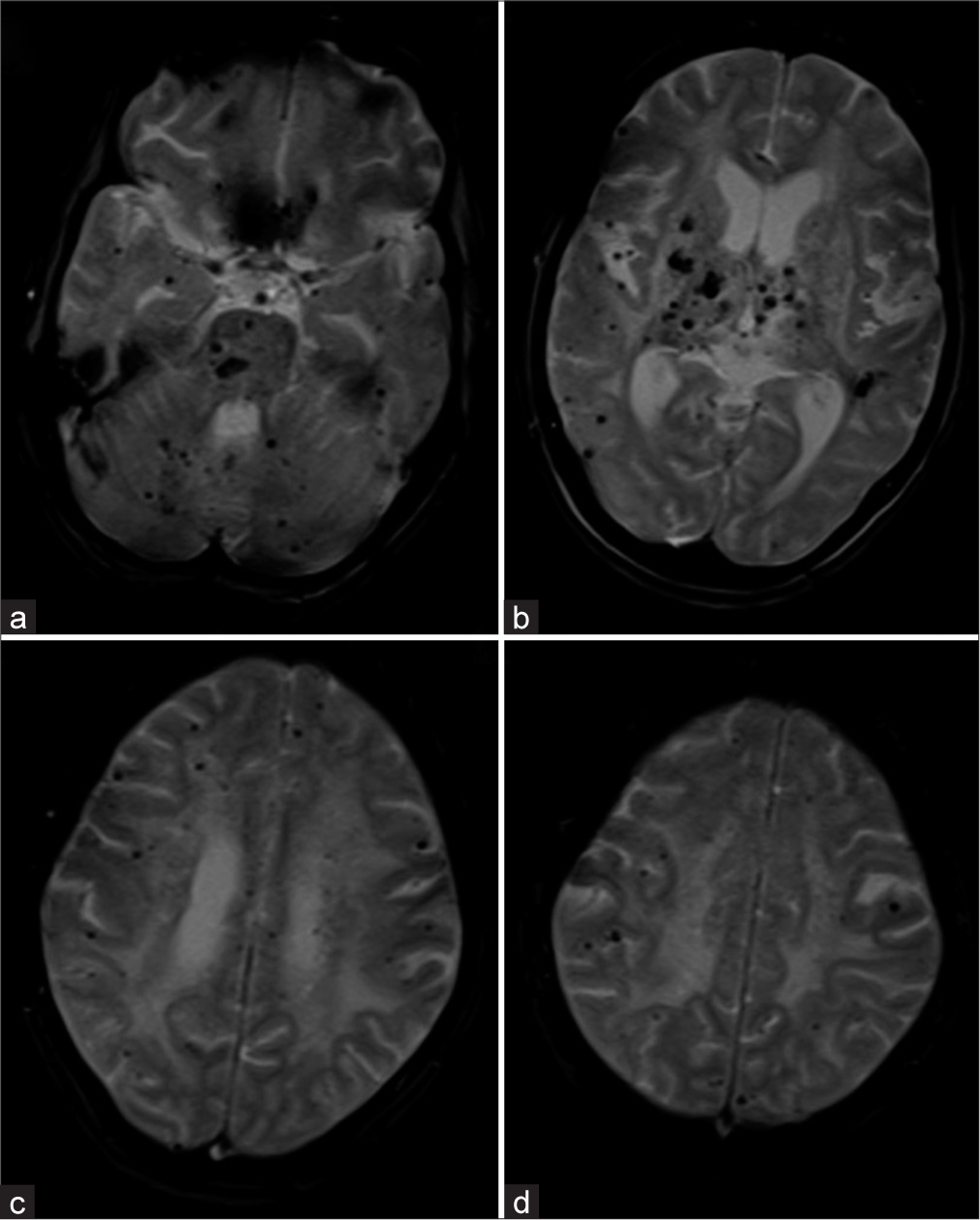
- (a-d) The GRE T2* axial images showing Grade IV (>25) microbleeds of both deep and lobar location.
| Radiological profile | Number (percentage) |
Average no. of MBs |
|---|---|---|
| Deep | 90 (82.57) | 8.1 |
| Deep+cerebellar | 105 (96.33) | 10.2 |
| Cortical/lobar microbleeds | 91 (83.49) | 19.9 |
| Total | 109 | 26 |
| Microbleeds | ||
| Grade I | 15 (13.76) | 4.4 |
| Grade II | 25 (22.94) | 8.1 |
| Grade III | 36 (33.03) | 16.7 |
| Grade IV | 33 (30.28) | 60.3 |
MBs: Microbleeds
| Radiological profile | Number (percentage) |
|---|---|
| Cortical infarcts | 30 (27.52) |
| Macro hemorrhages | 32 (29.36) |
| Lacunar infarcts <5 | 22 (20.18) |
| >5 | 40 (36.7) |
| WM changes (Fazekas) | |
| Grade I | 28 (25.69) |
| Grade II | 28 (25.69) |
| Grade III | 18 (16.51) |
| MRA findings – Dolichoectasia | 26 (23.85) |
| Vessel narrowing/occlusion | 7 (6.42) |
| MRA variants – Hypoplastic vertebral artery, thin vertebrobasilar |
28 (25.69) |
| Fetal PCA | 19 (17.43) |
MRA: Magnetic resonance angiography, PCA: Posterior cerebral artery, WM: White matter
The association of the MBs with a clinicoradiological profile including comorbidity factors was attempted using the Chi-square test/Fisher exact test. There was a statistically significant difference in number of deep MBs between hypertensive and non-hypertensive patients (P < 0.05). However, a comparison of the number of total or lobar cerebral MBs between hypertensive and non-hypertensive showed no statistically significant difference between the two groups. However, hypertension also showed significant association with lacunar infarcts (P < 0.05) and macrohemorrhages (P < 0.05) suggesting the common denominator small-vessel disease.
Similarly comparison of the number of cerebral MBs (lobar, deep, and total) between patients with lacunar infarcts and without lacunar infarcts showed no statistically significant difference between the two groups. Furthermore, a comparison of the number of cerebral MBs (lobar, deep, and total) between patients with various grades of leukoaraiosis did not show any statistically significant association. However, patients with Grade 3 leukoaraiosis were having more of Grade 4 cerebral MBs.
DISCUSSION
Nearly 5–6% of normal elderly are estimated to harbor cerebral MBs.[7,8] It may be slightly more in the Asian population both hypertensive and non-hypertensive.[9-11] Cerebral MBs are considered markers of not only for bleeding risk in those with macrobleeds or undergoing antithrombotic/anticoagulant treatment but also as a marker of “diffuse microangiopathy.”[9] There are not many studies about their incidence or association in our population. We tried to analyze the associated factors in those patients diagnosed with MBs on MR neuroimaging. Fazekas et al.[1] first described the histopathologic corroboration of GRE T2* hypointense foci as MBs and also found lipofibrohyalinosis in the underlying vessels. Greenberg et al.[2] refined the criteria so as to avoid the normal structures and other causes of such hypointense foci mimicking MBs. Later, these criteria were incorporated into standards for cerebral “small vessel disease.”[4] There have been attempts to evolve scoring scales for the MBs such as the MB anatomical rating scale and the brain observer MB scale.[12,13] The former uses each brain region – four lobes, cerebellum, brainstem, thalami, basal ganglia, internal and external capsule and insula – and the latter method divided them as lobar (cortical and subcortical white matter) and deep (basal ganglia and capsular tracts) for scoring.
MRI technique also has been shown the influence the visualization of MBs like 1.5 Tesla or 3T MRI, Gradient echo T2* imaging or the SWI, 3D acquisition of sub-millimeter spatial resolution with flow compensation, thinner sections (<5 mm), longer echo time (TE)/repetition time (TR), parallel imaging technique, and use of phase map in SWI and they are now part of standard sequence to improve the detection of MBs.[14-17]
The mimics of MBs include those with blood or blood degradation products (such as vessels and small type IV cavernomas)[2,18] and those without blood products such as calcifications, some micrometastases (such as melanoma).
The lobar and deep MBs have different clinical risk factors. The lobar is associated mainly with CAA including a stronger relation to the hereditary Dutch type of CAA. Other hereditary disorders featuring MBs include cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)/cerebral autosomal-recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL), COL4A mutations, Fabry disease, Pompe disease, Tangier disease, retinal vasculopathy with cerebral leukodystrophy, and pseudoxanthoma elasticum.[3,19]
Lobar MBs have been shown to be an independent risk factor for cognitive impairment with higher MBs in baseline associated with risk of functional dependence with an odds ratio of 1.16 for every further MB.[20] Although the parieto-occipital region is the common location for lobar MBs in CAA, those in the frontal lobe and caudate are more associated with cognitive/executive impairment.[21] In fact, the lobar MBs are considered the link between amyloid and neurovascular factors of Alzheimer’s disease. MBs also are important in those patients developing inflammatory syndrome of Amyloid-related imaging abnormalities (ARIA) and MBs more than 4 are considered a contraindication for anti-ARIA treatment using drugs such as bapineuzumab.[22]
The clinical associations for deep MBs include that of similar risk factors that are associated with other imaging markers of small-vessel disease such as hypertension, diabetes, dyslipidemia, smoking, apolipoprotein E genotype, and alcoholism apart from low cholesterol and atorvastatin use.[4,17,23] Although cerebellar and brainstem MBs are considered part of deep MBs, cerebellar superficial cortical MBs are also noted in CAA. In our study, the risk factor distribution is hypertension (62.39%), diabetes (23.85%), smoking (9.17%), and alcoholism (31.19%).
There are some locations considered relatively specific for underlying disorder like thalami in CADASIL, occipital in post-radiation, frontal and corpus callosal in post-trauma, and periventricular and temporal regions in Moyamoya disease.[24] The other acquired disorders reported with MBs include traumatic brain injury (linear, corpus callosal involvement), uremia on dialysis (irrespective of mode of dialysis or heparin use), fat embolism, cardiac myxoma or valve replacement, post-carotid stenting, infective endocarditis, radiation therapy (particularly whole-brain of more than 25 Gy), and those on ECMO support and COVID-19 infection.[25,26] The radiation-induced MBs may partly benefit from anti-angiogenic therapy.[27] However, MBs have been shown to be less in those with TIAs than in complete ischemic stroke and those with cortical infarcts.[5,21] The associations in our study group are head injury (4.6%), previous neurosurgery (4.6%), radio-/chemo-therapy (8.3%), cardiovascular including valvular/infective (12.8%), anti-platelet/anticoagulant use (15.6%), previous TIAs/stroke (9.2%), and uremia (5.5%). Hypertension showed significant association with deep cerebral MBs. It is possible that there are fewer patients with head injury since MRI is not routinely used for the evaluation of trauma cases.
Imaging of cerebral MBs should at least give a semi-quantitative estimate (<5, 5–10, >10 or similar) and mention the numbers as they might have prognostic and management implications.[6] The risk of further recurrent bleed increases with the baseline number of MBs.[28] However, as per recent guidelines, MBs of <10 are not considered sufficient reason to withhold anti-thrombotic treatment in otherwise deserving/eligible patients with ischemic stroke and no recommendation for routine MRI before starting intravenous antithrombotic treatment.[29] Our study showed average bleed in those mainly with deep and deep with cerebellar MBs was 8–10, but it was nearly 20 in those with lobar MBs.
CONCLUSION
In this descriptive study, 109 patients diagnosed with cerebral microbleeds on MR neuroimaging were analyzed for clinical predisposing factors and categorized by location (lobar, deep, cerebellar) and number (<5, 5 – 10, 11 – 25 and >25). The co-existing abnormalities that are also markers of small vessel disease like lacunar infarcts and white matter leukaraiosis were evaluated apart from cortical infarcts and macrobleeds. Vascular abnormalities and variants were also noted for association. Further structured studies with large sample population in each of the subcategory and risk factor may reveal more insights and better understanding of this important evolving marker of cerebral small vessel disease.
Ethical approval
The research/study approved by the Institutional Review Board at Institutional Ethics Committee (Human studies) JIPMER Puducherry, number JIP/IEC/2017/0396, dated May 26, 2018.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: Evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637-42.
- [Google Scholar]
- Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009;8:165-74.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds: A guide to detection and clinical relevance in different disease settings. Neuroradiology. 2013;55:655-74.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822-38.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and risk factors of cerebral microbleeds: The Rotterdam scan study. Neurology. 2008;70:1208-14.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. 2009;72:171-6.
- [CrossRef] [PubMed] [Google Scholar]
- MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991-4.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical associations of cerebral microbleeds on magnetic resonance neuroimaging. J Stroke Cerebrovasc Dis. 2014;23:2489-97.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds are common in ischemic stroke but rare in TIA. Neurology. 2005;65:1914-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds on MRI: Prevalence, associations, and potential clinical implications. Neurology. 2006;66:165-71.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of cerebral microbleeds in the East and West: Individual participant meta-analysis. Neurology. 2019;92:e1086-97.
- [CrossRef] [PubMed] [Google Scholar]
- The Microbleed anatomical rating scale (MARS): Reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759-66.
- [CrossRef] [PubMed] [Google Scholar]
- improving interrater agreement about brain microbleeds: Development of the Brain Observer MicroBleed Scale (BOMBS) Stroke. 2009;40:94-9.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging detection of cerebral microbleeds: Effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338-43.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical relevance of improved microbleed detection by susceptibility-weighted magnetic resonance imaging. Stroke. 2011;42:1894-900.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds: from depiction to interpretation. J Neurol Neurosurg Psychiatry. 2021;92:598-607.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of calcification with MRI using susceptibility-weighted imaging: A case study. J Magn Reson Imaging. 2009;29:177-82.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleed mimics In: Werring DJ, ed. Cerebal microbleeds: Pathophysiology to clinical practice. Cambridge: Cambridge University Press; 2011. p. :44-8.
- [CrossRef] [Google Scholar]
- Cerebral microbleeds: A magnetic resonance imaging review of common and less common causes. Eur J Neurol. 2018;25:441-50.
- [CrossRef] [PubMed] [Google Scholar]
- Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: A two-centre cohort study. Brain. 2006;129:2375-83.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265-75.
- [CrossRef] [PubMed] [Google Scholar]
- Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: A retrospective analysis. Lancet Neurol. 2012;11:241-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds in the population based AGES-Reykjavik study: Prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of asymptomatic microbleeds in patients with moyamoya disease. Neurol Med Chir (Tokyo). 2005;45:495-500.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral microbleeds and their influence on cognitive impairment in Dialysis patients. Brain Imaging Behav. 2021;15:85-95.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid formation of cerebral microbleeds after carotid artery stenting. Cerebrovasc Dis Extra. 2012;2:9-16.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of anti-angiogenic therapy on the formation of radiation-induced microbleeds in normal brain tissue of patients with glioma. Neuro Oncol. 2016;18:87-95.
- [CrossRef] [PubMed] [Google Scholar]
- Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255:1679-86.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344-418.
- [CrossRef] [Google Scholar]







