Translate this page into:
Clinical characteristics and imaging patterns of cerebral infarction with outcomes of carotid artery stenting in symptomatic carotid stenosis: An eight-year journey
*Corresponding author: Sripadma PV, Department of Neurology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India. sripadma.pv@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Pai AR, Ramachandran P, Rai A, Sripadma PV. Clinical characteristics and imaging patterns of cerebral infarction with outcomes of carotid artery stenting in symptomatic carotid stenosis: An eight-year journey. J Neurosci Rural Pract. 2024;15:468-76. doi: 10.25259/JNRP_627_2023
Abstract
Objectives:
Carotid artery stenting (CAS) for symptomatic carotid stenosis (SCS) has emerged as an attractive option in recent times. CAS and topographical patterns of stroke in symptomatic stenosis have been inadequately addressed. With this objective, we conducted a study to analyze infarct patterns and complications of carotid stenting and determine outcome predictors after stenting.
Materials and Methods:
A single-center retrospective study from January 01, 2015, to December 31, 2022, on patients with SCS, who underwent carotid stenting with at least six months of follow-up was conducted. Infarct patterns, angiographic findings, procedural complications, and outcomes (favorable [modified Rankin scale (mRS) ≤2] or unfavorable [mRS >2]) were recorded. Chi-square, analysis of variance for qualitative and quantitative variables was employed. Significant variables on univariate analysis were entered into regression and outcome predictors were determined.
Results:
Ninety-six records were included in the study. Forty-six (47.91%), 12 (12.50%), and 38 (39.58%) patients had territorial infarcts (TIs), border-zone infarcts (BZIs), and mixed infarcts (MIs). National Institutes of Health Stroke Scale (NIHSS) <5 and transient ischemic attack (TIA) were significant (P < 0.05) while the circle of Willis anomalies were comparable in TI versus BZI versus MI. Bradycardia (54.16%) and vessel spasm (19.79%) were noted during stenting. Successful revascularization (residual stenosis <20%) was achieved in 97.87%. Procedural complications were comparable in TI versus BZI versus MI. Minor cerebral hyperperfusion syndrome (CHS) with headache (9.57%), seizure (2.12%) peaking between 6 and 12 h, and severe with basal ganglia hemorrhage and death occurred. There were no major strokes. 64.13% and 75.28% achieved a favorable mRS at one and six months, respectively. NIHSS ≤10, early intervention (≤ 2 weeks), absence of diabetes, hypertension, or ischemic heart disease were significant (P < 0.05) for a favorable outcome. NIHSS ≤10, absence of hypertension at one month and NIHSS ≤10, absence of diabetes at six months were predictors of a favorable outcome.
Conclusion:
Severe carotid disease predisposed to CHS. Overall, CAS was a safe and effective procedure with 74.15% achieving favorable outcomes at six months.
Keywords
Ischemic stroke
Carotid stenosis
Stenting
Hyperperfusion
INTRODUCTION
Stroke constitutes the fourth leading cause of mortality and the fifth leading cause of death in India.[1] Extracranial carotid atherosclerosis contributes to 18–25% of ischemic strokes worldwide.[2] Symptomatic carotid stenosis (SCS) predisposes to high recurrence rates of ischemic stroke and merits immediate management.[3,4] The treatment for SCS has gained clarity in recent times with optimal medical management for all patients and additional carotid artery stenting (CAS) or endarterectomy for those with more than 50% stenosis.[3,4] Less invasiveness, shorter post-operative recovery time, and the growing availability of interventionists surely make carotid stenting a more attractive option.[3,4]
Topographical patterns of border-zone infarcts (BZI) and territorial infarcts (TIs) stand described with BZIs having more seizures, transcortical aphasia, TIA, and anomalous circle of Willis.[5,6] Carotid endarterectomy (CEA) was previously reported to be safer with fewer complications in patients with TI than with BZI.[7]
Patterns of cerebral infarction in symptomatic carotid disease and the outcomes of carotid stenting are inadequately addressed in Indian studies. Keeping this in mind, we compiled our 8-year data on patients with SCS who underwent carotid stenting and explored their clinical and imaging characteristics, peri-procedural complications, and predictors of outcome after stenting.
MATERIALS AND METHODS
Research design and ethics
A retrospective analysis of patients enrolled in our carotid stenting registry as per STROBE guidelines was performed. Patients with carotid stenting done for SCS and at least six months of follow-up between January 1st, 2015, and December 31st, 2022 were included in this study. Their demographic details, infarct patterns, and peri and post-procedural complications with short and long-term outcomes at one and six months after the procedure were recorded. CAS or endarterectomy was offered to patients with SCS based on the current guidelines.[4,8] Diagnostic invasive angiography and carotid stenting procedures were uniform. The institute’s ethics committee approval was taken vide letter no. IEC 23/2023 and the Helsinki principles were followed.
Selection and defining the groups
Patients who underwent carotid stenting for symptomatic carotid stenting with at least six months of follow-up were included in the study. Patients with incomplete medical records, pregnant women, and patients with very severe strokes (National Institutes of Health Stroke Scale [NIHSS] ≥20) were not considered for stenting and were excluded from the study.
Symptomatic carotid artery stenosis refers to sudden onset focal neurological symptoms attributable to ipsilateral carotid atherosclerotic disease, including one or more ischemic strokes or transient ischemic attack within the previous six months.[4] Both the European Society of Cardiology and American Heart Association/American Stroke Association guidelines currently recommend optimal medical management with antiplatelet and statins for all patients with SCS. Interventional management with CEA or CAS is recommended for >50% stenosis when the estimated periprocedural complication rate (death or stroke) is <6% for the latter and preferably performed within 14 days of the ictus.[3,4] CAS emerged as an alternative to the gold standard CEA in 1989. In a recently conducted meta-analysis, both CAS and CEA had comparable rates of periprocedural stroke, myocardial infarction, and death.[9]
Patients were divided into three groups based on the topographical pattern of infarcts on imaging [Figure 1]. BZIs occur at the junction of two neighboring arterial territories. Two main patterns of infarction are described under BZI: cortical border-zone infarct (CBZ) and internal BZI.[10] The CBZ are further subdivided into anterior and posterior infarcts, with anterior infarcts at the junction of anterior and middle cerebral arteries and posterior BZI between the middle and posterior cerebral arteries (PCAs). The internal BZI are sub-cortical at junctions of anterior choroidal, recurrent artery of Heubner, and lenticulostriate arteries territories with the anterior, middle, and PCA territories.[11] These zones are highly vulnerable to infarction due to their inherent lower perfusion pressure, which is compounded by a severe proximal arterial stenosis resulting in a hemodynamic infarct.
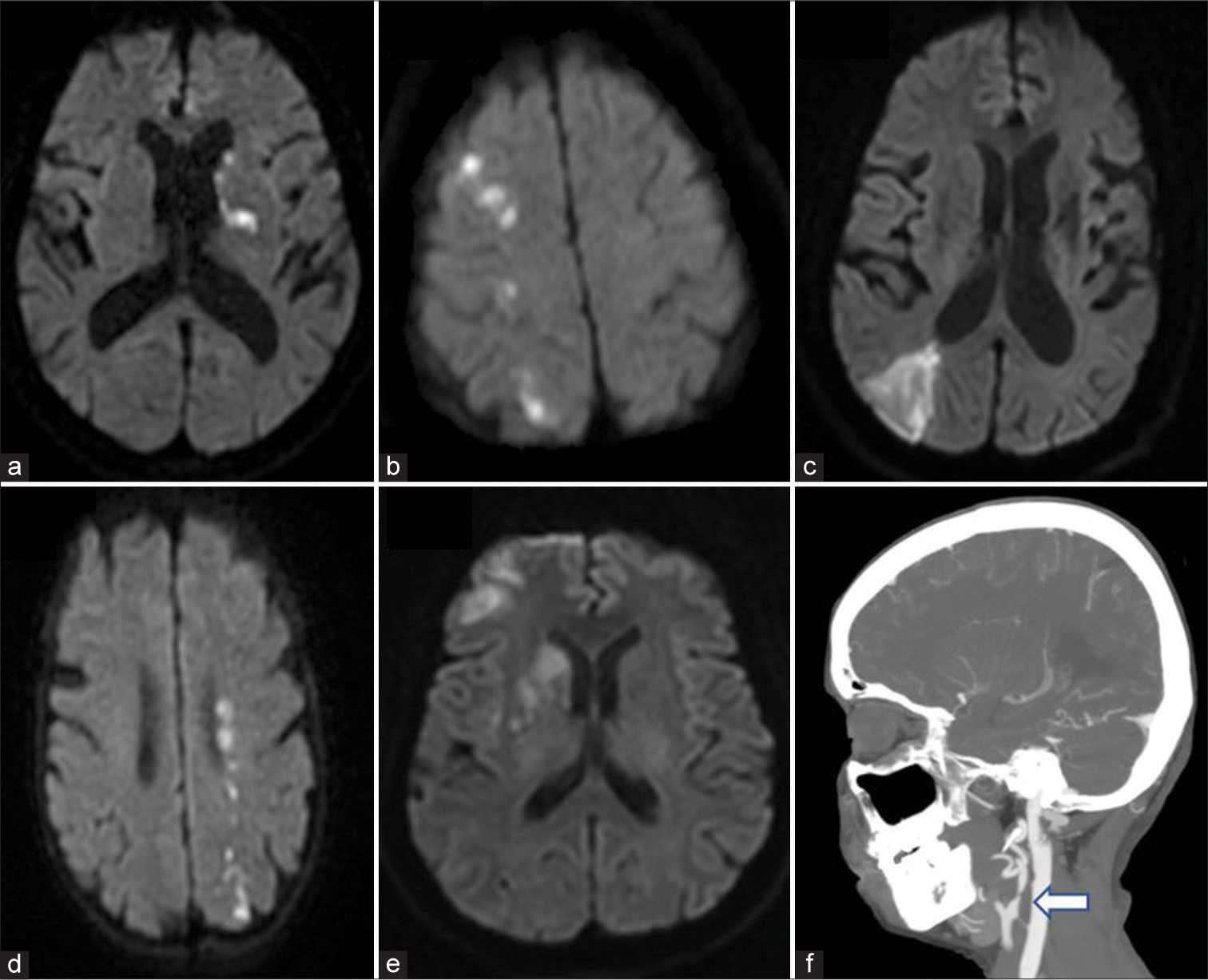
- (a) Territorial infarct in left middle cerebral artery (MCA) region, (b) Right anterior cerebral artery (ACA)-MCA cortical and internal border zone, (c) Right MCA-posterior cerebral artery cortical border zone infarct (BZI), this patient had a mixed infarct pattern, (d) Mixed infarct: Internal and posterior cortical BZI, (e) Right MCA sub-cortical and ACA-MCA cortical border zone, (f) Computed tomography angiogram showing severe carotid stenosis of the left internal carotid (white arrow).
An anterior circulation infarct not involving the topography of the described BZI was considered as TI and infarcts with involvement of both these regions (TI and BZI) were categorized as mixed infarcts (MIs) in this study.
Periprocedural details
Written informed consent was taken and an adequate dose of dual antiplatelets was given before the procedure (aspirin 150 mg and clopidogrel 75 mg for at least the previous 5 days or a loading dose of aspirin 325 mg and clopidogrel 300 mg at least 12 h before the procedure if antiplatelet therapy had not been administered previously). Diagnostic angiography was done pre-procedurally to assess the lesion severity and vascular anatomy. This was crucial in identifying technically challenging lesions with excessive vascular tortuosity and heavy calcification. If the lesion appeared to predispose to increased procedural risk, CEA was offered. Femoral access was taken and patients were adequately heparinized with an activated clotting time of 200–250 s maintained throughout the procedure. The embolic protection device (EPD) was delivered distal to the stenotic segment using a 0.014” guide wire of the EPD system. Self-expanding nitinol stents were employed routinely with the stent diameter being 1–2 mm more than the maximum diameter of the diseased segment (usually a length of 40–60 mm and diameter of 6–8 mm). A check angiogram was done after stent deployment to look for contrast transit across the stent and residual stenosis if any. Technical success was defined as residual stenosis ≤20% and establishing Grade 3 Thrombolysis in Cerebral Infarction (TICI) antegrade flow.[4] Intravenous atropine (0.6–1.2 mg) was used if bradycardia occurred on balloon inflation or stent placement in the common carotid artery.
Data collection and measurements
All patients underwent routine blood work-ups with complete hemograms, renal function tests, liver function tests, blood sugar, glycosylated hemoglobin, and lipid profile. Stroke evaluation was done with magnetic resonance imaging of the brain, computed tomography (CT) or magnetic resonance-based angiography of the extra and intracranial vessels, carotid and vertebral artery Doppler with electrocardiogram, echocardiogram, and 72 h of Holter monitoring. The variables for comparison between the groups (TI vs. BZI vs. MI) were age, sex, vascular risk factors such as hypertension, diabetes, dyslipidemia (defined as the presence of any of these: Total low-density cholesterol >100 mg/dL, high-density lipoprotein <50 mg/dL, triglycerides >150 mg/dL), current smoking or tobacco chewing, ischemic heart disease, baseline NIHSS, previous TIA, cerebral angiogram findings.[12,13] In addition, we listed the peri-procedural complications. We compared the one- and six-month outcomes of carotid stenting using the previously mentioned variables with a modified ranking scale (favorable [modified Rankin scale (mRS) ≤2] versus unfavorable [mRS >2]) and estimated the predictors of a favorable outcome.[14]
Statistical analyses
Qualitative variables were described as numbers and/or percentages and compared between the two groups using the Chi-square or Fisher’s exact test. Quantitative variables were described as means and standard deviations and compared using analysis of variance. Variables with a significant P < 0.05 were entered into the multivariate binary logistic regression model for the calculation of outcome predictors with 95% confidence intervals. Statistical analysis was done using Graphpad Prism version 9.0 and SPSS V.24 (IBM Corp; Armonk, New York, USA).
RESULTS
Ninety-six cases of CAS done for SCS with a minimum of six months of follow-up were included between 2015 and 2022. There were 46 (47.91%), 12 (12.50%), and 38 (39.58%) patients in the territorial, border-zone, and MI groups, respectively. No significant differences existed among the groups in mean age, gender, vascular risk factors such as hypertension, diabetes, dyslipidemia, current smoking or tobacco chewing, and ischemic heart disease [Table 1]. Among the 12 patients in BZI, 2 (16.66%) had isolated internal BZI, and 10 (83.33%) had a combination of both internal and cortical BZIs. BZI patients had significantly more minor strokes with NIHSS <5 (P = 0.01) and none had NIHSS >15 [Table 1]. Transcortical aphasia (sensory, motor, or mixed) was observed in 25% (3/12) BZI while it was 6.52% (3/46) and 10.52% (4/38) in territorial and MIs groups, respectively. TIA was significant in BZI (P = 0.009). Circle of Willis anomalies, percentage of stenosis, treated vessel, and bilateral disease were comparable [MI vs. BZI vs. TI, Table 2]. Transient bradycardia (52/96, 54.16%) was commonly encountered during the stenting procedure, and vessel spasm (19/96, 19.79%) and transient hypotension necessitating inotrope use (11/96, 11.45%) were also noted [Table 3]. Technical success of revascularization (TICI 3 or residual stenosis <20%) was achieved in 97.87% (92/94) cases [Figures 2a-c]. Peri-procedural (<30 days) death rate was 4.1% [4/96, Table 3]. Two (2.08%, 2/96) patients with multiple comorbidities (diabetes, hypertension, and ischemic heart disease) died of cardiac arrest shortly after stenting. Two (2.12%, 2/94) other deaths occurred, one attributable to cerebral hyperperfusion syndrome (CHS) (basal ganglia hemorrhage with raised intracranial pressure) and the other to myocardial infarction [Table 3] in the 1st week after stenting. Subsequently, between one to six months after stenting three (3.26%, 3/92) deaths occurred. One is secondary to cardiac arrhythmia and two attributable to multiorgan dysfunction in patients with multiple vascular risk factors [Table 3]. We also compared outcomes (mRS ≤2 vs. mRS >2) at one and six months after stenting using the previously mentioned variables and dichotomizing NIHSS at 10. NIHSS ≤10, early intervention (≤2 weeks after stroke), and absence of hypertension, diabetes, and ischemic heart disease were associated with favorable outcomes at the end of one month on univariate analysis [Table 4]. After six months, low NIHSS, early intervention, and absence of hypertension and diabetes were associated with a good outcome [Table 4]. Multivariate binary logistic regression analysis was applied to these significant variables. NIHSS ≤10 and absence of hypertension (P = 0.03, 0.04) at 1 month and absence of diabetes with low NIHSS (P = 0.04, 0.01) were predictors of favorable outcomes at one and six months, respectively [Table 5].
| Total n=96 | Territorial infarcts 46 (47.91%) n (%) |
Border-zone infarcts 12 (12.50%) n (%) |
Mixed infarcts 38 (39.58%) n (%) |
P-value |
|---|---|---|---|---|
| Age (years) (Mean±SD) | 66.80±5.87 | 67.08±6.62 | 65.97±6.69 | 0.786 |
| Male | 34 (73.91) | 8 (66.66) | 21 (55.26) | 0.258 |
| Hypertension | 30 (65.21) | 7 (58.33) | 31 (81.57) | 0.131 |
| Diabetes | 36 (78.26) | 6 (50.00) | 22 (57.89) | 0.089 |
| Ischemic heart disease | 19 (41.30) | 2 (16.66) | 16 (4.21) | 0.286 |
| Dyslipidemia | 40 (86.95) | 9 (75.0) | 30 (78.94) | 0.636 |
| Smoking | 23 (50.00) | 5 (41.66) | 21 (55.26) | 0.533 |
| Seizures | 6 (13.04) | 2 (16.66) | 4 (10.52) | 0.847 |
| Previous TIA | 5 (10.86) | 6 (50.00) | 8 (21.05) | 0.009* |
| NIHSS<5 | 9 (19.56) | 7 (58.33) | 8 (21.05) | 0.015* |
| NIHSS 5–15 | 33 (71.73) | 5 (41.66) | 27 (71.05) | 0.118 |
| NIHSS 16–20 | 4 (8.69) | 0 | 3 (7.89) | 0.583 |
| Transcortical Aphasia | 3 (6.52) | 3 (25) | 4 (10.52) | 0.166 |
TIA: Transient ischemic attack, NIHSS: National Institutes of Health Stroke Scale, n: Number of observations, SD: Standard deviation
| Total n=96 | Territorial infarcts 46 (47.91%) n (%) |
Border-zone infarcts 12 (12.50%) n (%) |
Mixed infarcts 38 (39.58%) n (%) |
P-value | |
|---|---|---|---|---|---|
| Circle of Willis anomalies | |||||
| Hypoplastic vertebral artery | 12 (26.08) | 3 (25.00) | 11 (28.94) | 0.92 | |
| Hypoplastic anterior cerebral artery | 13 (28.26) | 2 (16.66) | 11 (28.94) | 0.69 | |
| Fetal posterior cerebral artery | 7 (15.21) | 3 (25.00) | 10 (26.31) | 0.39 | |
| Combination of above | 4 (8.69) | 2 (16.66) | 5 (13.15) | 0.65 | |
| Others | |||||
| Right internal carotid artery (Treated vessel) | 27 (58.69) | 5 (41.66) | 24 (63.15) | 0.42 | |
| 50–69% stenosis | 4 (8.69) | 1 (8.33) | 5 (4.16) | ||
| 70–99% stenosis | 42 (91.30) | 11 (91.66) | 33 (86.84) | 0.87 | |
| Asymptomatic carotid | 3 (6.52) | 1 (8.33) | 2 (5.26) | 0.92 | |
| Contralateral carotid occlusion | 2 (4.34) | --- | 1 (2.63) | 0.73 | |
n: Number of observations
| Total n=96 | Complications | n (%) |
|---|---|---|
| Intra-procedure | Bradycardia requiring atropine | 52 (54.16) |
| Vessel spasm | 19 (19.79) | |
| Inotrope use | 11 (11.45) | |
| Revascularization success | 92 (97.87) | |
| Peri-procedural deaths Immediate |
Cardiac arrest and death | 4 (4.16) 2 (2.08) |
| <1 month | Cerebral hyperperfusion syndromemyocardial infarction | 1 (1.06) 1 (1.06) |
| Post-procedure Cerebral hyperperfusion syndrome (CHS) | Severe headache | 9 (9.57) |
| Seizures | 2 (2.12) | |
| Hemorrhage (basal ganglia) | 1 (1.06) | |
| Altered mentation | 3 (3.19) | |
| Increased vasogenic edema | 1 (1.06) | |
| Minor stroke/TIA: <1 month | Ipsilateral | 2 (2.12) |
| Contralateral | --- | |
| Ipsilateral central retinal artery occlusion | 1 (1.06) | |
| >1 month----<6 months | Ipsilateral | --- |
| Contralateral | 2 (2.17) | |
| Death>1 month | Cardiac arrhythmia | 1 |
| Multi-organ dysfunction | 2 |
TIA: Transient ischemic attack, n: Number of observations
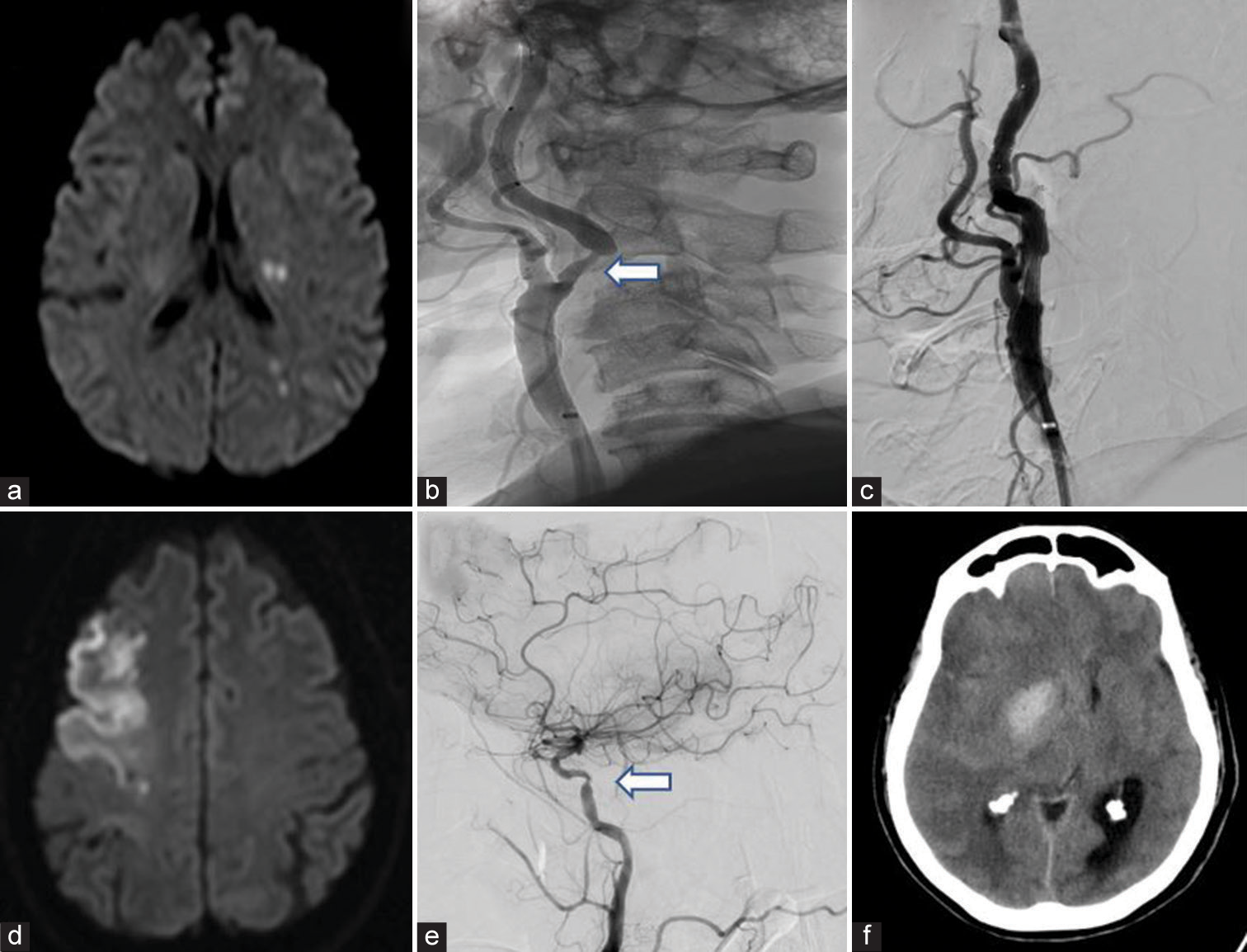
- (a) Mixed infarct-Left middle cerebral artery (MCA) TI with MCA- posterior cerebral artery border zone infarct, (b) Pre-stenting digital subtraction angiography (DSA) showing severe >70% stenosis (white arrow), (c) Post-stent deployment DSA showing no residual stenosis, (d) territorial infarct in right MCA, (e) DSA showing tight stenosis of right internal carotid artery (white arrow), (f) Cerebral hyperperfusion syndrome – Basal ganglia hemorrhage with mass effect and vasogenic edema in same patient post-stenting.
| Favorable mRS≤2 59 (64.13%) At 1 month n (%) |
Unfavorable mRS>2 33 (35.86%) At 1 month n (%) |
P-value | Favorable mRS≤2 6 (75.28%) At 6 months n (%) |
Unfavorable mRS>2 22 (24.71%) At 6 months n (%) |
P-value | |
|---|---|---|---|---|---|---|
| Male | 40 (67.79) | 20 (60.60) | 0.487 | 44 (65.67) | 14 (63.63) | 0.862 |
| Female | 19 (32.20) | 13 (39.39) | 22 (32.83) | 9 (40.90) | ||
| Age≥80 | 2 (3.38) | 3 (9.09) | 0.247 | 2 (2.98) | 2 (9.09) | 0.230 |
| Age≥70 | 22 (37.28) | 12 (36.36) | 0.929 | 27 (40.29) | 6 (27.27) | 0.272 |
| Hypertension | 35 (59.32) | 30 (90.90) | 0.001* | 45 (67.16) | 17 (77.27) | 0.370 |
| Diabetes | 33 (55.93) | 29 (87.87) | 0.001* | 39 (58.20) | 20 (90.90) | 0.004* |
| Ischemic heart disease | 16 (27.11) | 18 (54.54) | 0.009* | 20 (29.85) | 13 (59.09) | 0.013* |
| Dyslipidemia | 47 (79.66) | 28 (84.84) | 0.416 | 55 (82.08) | 18 (81.81) | 0.977 |
| Smoking | 32 (54.23) | 13 (39.39) | 0.171 | 30 (44.77) | 13 (59.09) | 0.243 |
| Seizures | 7 (11.86) | 4 (12.12) | 0.971 | 8 (11.94) | 3 (13.63) | 0.833 |
| Previous TIA | 13 (22.03) | 5 (15.15) | 0.424 | 12 (17.91) | 5 (22.72) | 0.618 |
| ≤2 weeks after stroke stenting | 49 (83.05) | 20 (60.60) | 0.017* | 55 (82.08) | 13 (59.09) | 0.027* |
| NIHSS≤10 | 55 (93.22) | 16 (48.48) | <0.000* | 60 (89.55) | 9 (4.09) | <0.001* |
TIA: Transient ischemic attack, NIHSS: National Institutes of Health Stroke Scale, mRS: Modified Rankin scale, n: Number of observations, *significant
| At 1 month | B | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|---|
| Normotension | −1.062 | 0.346 | 0.130–0.918 | 0.033* |
| Euglycemic | −0.163 | 0.849 | 0.314–2.296 | 0.747 |
| No ischemic heart disease | 0.159 | 1.173 | 0.447–3.074 | 0.746 |
| NIHSS≤10 | −1.426 | 0.240 | 0.061–0.939 | 0.040* |
| ≤2 weeks after stroke stenting | −0.064 | 0.938 | 0.319–2.755 | 0.907 |
| At 6 months | ||||
| Euglycemic | −1.068 | 0.344 | 0.119–0.992 | 0.048* |
| No ischemic heart disease | 0.535 | 1.707 | 0.594–4.902 | 0.321 |
| NIHSS≤10 | 1.432 | 4.188 | 1.347–13.023 | 0.013* |
| ≤2 weeks after stroke stenting | −0.339 | 0.712 | 0.196–2.587 | 0.606 |
NIHSS: National Institutes of Health Stroke Scale. *significant <0.05
DISCUSSION
Concurrent to previous studies, BZI patients had significantly more minor strokes (NIHSS <5, P = 0.01) in comparison to TI and MI.[3,15] In addition, transcortical aphasia (25% vs. 6.52 and 10.52 in TI and MI, respectively) was more common in BZI (though it did not reach statistical significance) akin to existing literature.[5] TIAs were significant in the BZI group (P = 0.009), similar to existing studies.[5] In contrast to Joinlambert et al., seizures had no predilection and were comparable among the groups (TI vs. BZI vs. MI, P = 0.84 and 0.87, respectively).[5] The pattern of BZI, isolated internal border-zone (2/12, 16.66%), and combined (internal and cortical border-zone) infarcts (10/12, 83.33%) was in harmony with the current literature, wherein, carotid stenosis associated hemodynamic infarcts were associated with IBZ and combined (IBZ with CBZ) pattern while cardioembolic had more of cortical BZIs.[6,15] No significant angiographic (circle of Willis or vertebral artery hypoplasia) differences were detected in BZI versus MI or TI. This agreed with ElGammal et al. and Szabo et al. and was in contrast to that of Kumral et al. who noted a higher incidence of a hypoplastic PCA in patients with BZI.[6,16,17]
The European trials of Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) showed more clinical events (death and disabling stroke) with CAS than CEA and the International Carotid Stenting Study randomized controlled trial showed more minor strokes with CAS.[18,19] Later, these trials were criticized for the non-utilization of EPDs and the limited experience of the interventionalists. Subsequently, short- and long-term outcomes of the Carotid Revascularization versus Endarterectomy Trial showed no difference in rates of myocardial infarction, stroke, or death between CEA and CAS.[20,21] In this study, 4.34% (4/92) suffered a minor stroke or TIA in the peri-procedural period (<30 days) which was comparable to the 5.7%, 4.4%, and 6.3% in SPACE, SAPPHIRE and EVA-3S studies, respectively.[18,22,23] A single case of acute visual loss due to central retinal artery occlusion occurred within 24 h of stenting in an elderly lady with bilateral severe carotid bifurcation disease and multiple vascular risk factors (hypertension, diabetes, and ischemic heart disease). Intraprocedural embolization of the ophthalmic artery, atherosclerosis of the culprit vessel, and anastomosis of the ophthalmic artery with external carotid artery branches were the possible mechanisms.[24]
Jean-Baptiste et al. and Kazandjian et al. previously reported procedural complications to be higher in BZI than in TI when these patients underwent CEA for SCS.[7,25] In light of this, we compared procedural complications of bradycardia, vessel spasm, and inotrope use among the groups (TI vs. BZI vs. MI) and found no significant differences. Cumulative death and stroke rates were 13% in BZI versus 0% in TI in Jean-Baptiste’s study.[25] In contrast, no deaths and a single TIA occurred in BZI against four deaths and 3 minor strokes (1 ipsilateral and 2 contralateral) in the TI and MI group in this study. No major strokes occurred in either group; however, a single case of CRAO occurred in the TI subset.
CHS comprises a clinical spectrum ranging from headache (most common) to seizures, altered mentation, new-onset neurological deficits, and life-threatening intra-cerebral hemorrhage (ICH) with raised intracranial pressure.[26] Single-photon emission CT and CT perfusion have been used for demonstrating CHS which is characterized by a >100% increase in cerebral blood flow of the revascularized region.[26] Hypertension and carotid stenosis of ≥90% along with poor collaterals are known to be risk factors for CHS.[26] We maintained post-procedural blood pressure at ≤140/90 mmHg to reduce the incidence of CHS.[26,27] Minor CHS, characterized by ipsilateral headache and seizure (9.57% and 2.12%, respectively) occurred, this agreed with the existing studies [Table 3][27] Ipsilateral headache peaked within 6–12 h of the procedure and improved with cerebral decongestants and stringent control of blood pressure. One patient became drowsy 12 h after the procedure but showed immediate recovery with anti-edema measures. However, two patients (2.17%) developed severe CHS, one resulting in death, and the other was discharged with mRS 4. The first patient had severe carotid disease (≥90% stenosis), postoperatively he developed severe headache, altered mentation, and worsening deficit, and imaging showed ipsilateral basal ganglia hemorrhage with midline shift necessitating an emergency decompressive hemicraniectomy. The second patient with a near total carotid occlusion developed altered mentation and imaging showed worsened vasogenic edema. Cerebral decongestants were started. Headache and mentation improved over the next 72 h, however, he had a poor outcome at discharge and after one and six months. Development of severe CHS in these patients agreed with the existing literature, wherein, most cases occurred secondary to severe carotid disease or contralateral carotid occlusion.[26,27] ICH occurring in the immediate post-procedure period could be secondary to the rupture of perforating arteries supplying the basal ganglia, consequent to the sudden normalization of the perfusion pressure [Figures 2d-f].[26,27]
Similar to Mihindu et al., we dichotomized NIHSS at 10 and analyzed the outcomes.[14] 64.13% (59/92) and 75.28% (67/89) of patients had favorable mRS 1 and 6 months after stenting. Of the 24.72% of patients with unfavorable mRS at the end of 6 months, the majority had mRS 3 and only those with severe strokes (3/89, NIHSS 16–20) had mRS 4. The absence of hypertension and NIHSS ≤10 after 1 month and the absence of diabetes, NIHSS ≤10 after 6 months were predictors of a favorable outcome [Table 5]. Patients with severe stroke (NIHSS ≥16) had more deaths in the immediate and one-month period after stenting (3/4, 75%). These cases also had multiple risk factors (diabetes, hypertension, ischemic heart disease). The AHA guidelines recommended early revascularization within 2 weeks of the ictus in 2006 and we tried to adhere to this (69/92, 75%).[28] Mihindu et al. reported an unfavorable outcome (mRS ≥3) at discharge when urgent (<48 h from stroke) carotid interventions (CEA or CAS) were performed.[14] No urgent interventions were performed in this study. Early intervention (≤2 weeks) was significant in the favorable mRS population at 1 and 6 months (0.003 and 0.027, respectively), though it was not a predictor of outcome.
Limitations and scope
Our study had a few limitations; this included the non-availability of carotid Doppler studies at the follow-up to look for restenosis. The evaluation of CHS was limited due to the restricted availability of perfusion studies. A longer follow-up would have shed more light on the complications; however, the fact that the deaths that occurred one month after stenting were not directly attributable to the procedure was reassuring.
CONCLUSION
With the majority of patients achieving a favorable outcome, shorter post-procedure recovery time, and few complications in trained hands CAS for SCS appear to be the procedure of choice in the near future. Furthermore, with more neurologists getting trained as neurointerventionalists, CAS may soon replace CEA at most centers with equally good outcomes. A close watch on the development of CHS in those with severe carotid disease is warranted.
Ethical approval
The research/study was approved by the Institutional Review Board at KASTURBA MEDICAL COLLEGE MANIPAL, number 23/2023, dated July 05, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Stroke in India: A systematic review of the incidence, prevalence, and case fatality. Int J Stroke. 2022;17:132-40.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559-72.
- [CrossRef] [PubMed] [Google Scholar]
- Management of extracranial carotid artery disease. Cardiol Clin. 2015;33:1-35.
- [CrossRef] [PubMed] [Google Scholar]
- Update in the treatment of extracranial atherosclerotic disease for stroke prevention. Stroke Vasc Neurol. 2019;5:65-70.
- [Google Scholar]
- Cortical border-zone infarcts: Clinical features, causes and outcome. J Neurol Neurosurg Psychiatry. 2012;83:771-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral border zone infarction: An etiological study. Egypt J Neurol Psychiatr Neurosurg. 2018;54:6.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of the type of cerebral infarct and timing of intervention in the early outcomes after carotid endarterectomy for symptomatic stenosis. J Vasc Surg. 2016;63:1256-61.
- [CrossRef] [PubMed] [Google Scholar]
- Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-53.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid artery stenting versus endarterectomy for stroke prevention: A meta-analysis of clinical trials. J Am Coll Cardiol. 2017;69:2266-75.
- [CrossRef] [PubMed] [Google Scholar]
- Border zone infarcts: Pathophysiologic and imaging characteristics. Radiographics. 2011;31:1201-14.
- [CrossRef] [PubMed] [Google Scholar]
- Internal borderzone infarction: A marker for severe stenosis in patients with symptomatic internal carotid artery disease. For the North American Symptomatic Carotid Endarterectomy (NASCET) Group. Stroke. 2000;31:631-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology, diagnosis, and management of dyslipidemia. Curr Probl Cardiol. 2006;31:445-86.
- [CrossRef] [PubMed] [Google Scholar]
- National Institutes of Health Stroke Scale: An alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke. 2020;51:282-90.
- [CrossRef] [PubMed] [Google Scholar]
- Patients with moderate to severe strokes (NIHSS score >10) undergoing urgent carotid interventions within 48 hours have worse functional outcomes. J Vasc Surg. 2019;69:1471-81.
- [CrossRef] [PubMed] [Google Scholar]
- Internal and cortical border-zone infarction: Clinical and diffusion-weighted imaging features. Stroke. 2006;37:841-6.
- [CrossRef] [PubMed] [Google Scholar]
- Acute stroke patterns in patients with internal carotid artery disease: A diffusion-weighted magnetic resonance imaging study. Stroke. 2001;32:1323-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of single and multiple borderzone infarct: Transcranial Doppler ultrasound/magnetic resonance imaging correlates. Cerebrovasc Dis. 2004;17:287-95.
- [CrossRef] [PubMed] [Google Scholar]
- Endarterectomy vs. angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial. Cerebrovasc Dis. 2004;18:62-5.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: The International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015;385:529-38.
- [CrossRef] [PubMed] [Google Scholar]
- The carotid revascularization endarterectomy versus stenting trial (CREST): Stenting versus carotid endarterectomy for carotid disease. Stroke. 2010;41(10 Suppl):S31-4.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016;374:1021-31.
- [CrossRef] [PubMed] [Google Scholar]
- Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial [published correction appears in Lancet Neurol 2009,8:135] Lancet Neurol. 2008;7:893-902.
- [CrossRef] [PubMed] [Google Scholar]
- Carotid endarterectomy in SAPPHIRE-eligible high-risk patients: Implications for selecting patients for carotid angioplasty and stenting. J Vasc Surg. 2004;39:958-66.
- [CrossRef] [PubMed] [Google Scholar]
- Ophthalmic artery occlusion after carotid revascularization. J Cerebrovasc Endovasc Neurosurg. 2013;15:326-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of preoperative border-zone (watershed) infarcts on the early postoperative outcomes of carotid endarterectomy after acute ischemic stroke. Eur J Vasc Endovasc Surg. 2013;45:210-7.
- [CrossRef] [PubMed] [Google Scholar]
- Update on cerebral hyperperfusion syndrome. J Neurointerv Surg. 2020;12:788-93.
- [CrossRef] [PubMed] [Google Scholar]
- Editor's choice - cerebral hyperperfusion syndrome after carotid artery stenting: A systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2018;56:322-33.
- [CrossRef] [PubMed] [Google Scholar]
- Timing of carotid revascularization procedures after ischemic stroke. Stroke. 2017;48:225-8.
- [CrossRef] [PubMed] [Google Scholar]







