Translate this page into:
Cervical Subependymoma: A rare case report with possible histogenesis
Address for correspondence: Dr. Manas Panigrahi, Consultant Neurosurgeon, Department of Neurosurgery, Krishna Institute of Medical Sciences (KIMS), #1-8-31/1 Ministers Road, Secunderabad – 500 003, Andhra Pradesh, India. E-mail: shyamsundar_krishnan@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Subependymomas are extremely rare lesions of the spinal cord. Only 33 cases including ours have been reported in the cervical cord. These are typically benign slow growing tumors occurring eccentrically within the cord, producing minimal neurological deficits. The clinical, radiological, and histopathological aspects of this unusual lesion have been reviewed in detail. As the histogenesis of this tumor is much debated, we propose an alternate origin for the same.
Keywords
Cervical subependymoma
histogenesis of subependymoma
spinal subependymoma
subependymoma
spinal tumor
Introduction
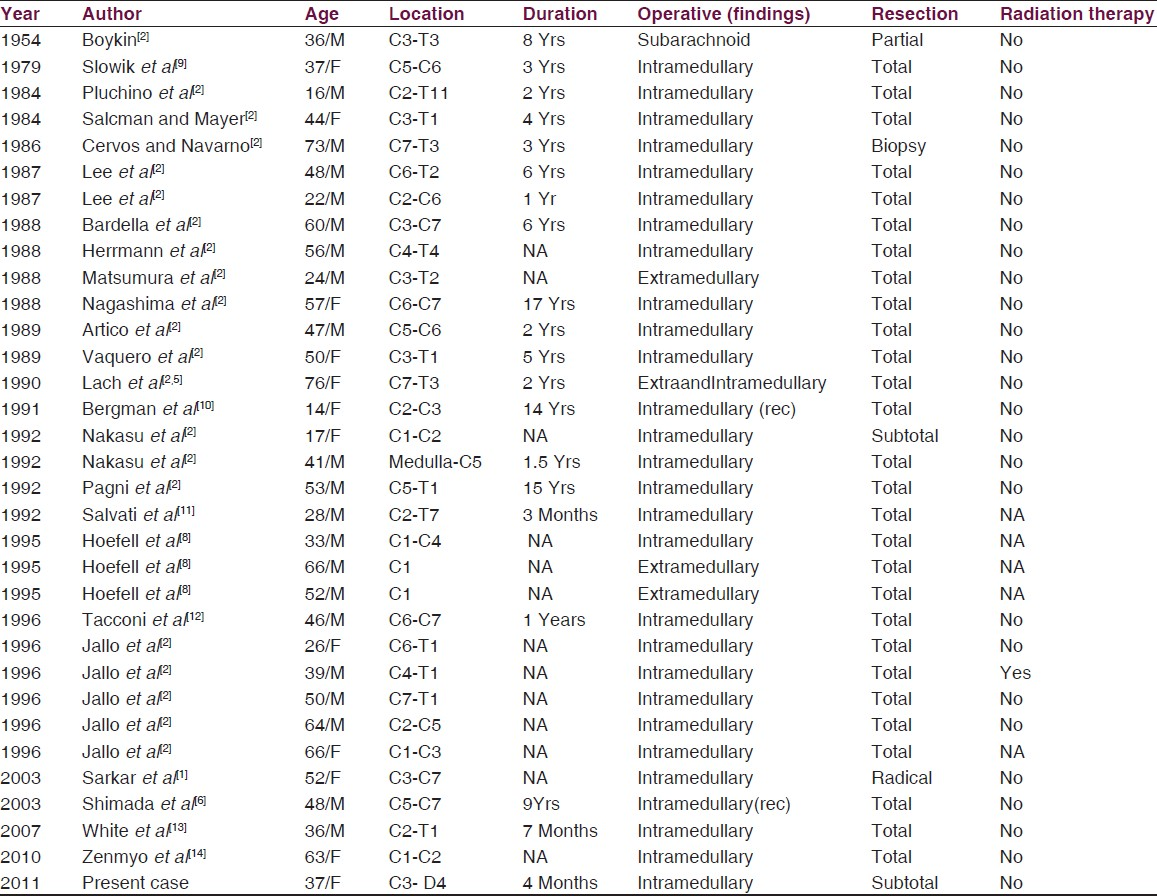
Subependymoma, first described by Scheinker in 1945, is a rare central nervous system tumor. Since its first description in the spinal cord by Boykin et al in 1954, only 47 cases have been reported. Out of these, 32 involved the cervical cord. In the spinal region, these lesions are predominantly subpial in location, with exophytic components. The location of these lesions in the spinal cord suggest a subpial glial progenitor origin rather than its proposed origin from subependymal progenitor cells.[1–4]
Case Report
A 37-year-old lady presented to us with complaints of neck pain and gait disturbances of 1 year duration with severe progressive weakness of her right upper limb since 3 months. She had a grade 3/5 power in her right upper limb with muscle wasting. Rest of her limbs had mild weakness with spasticity. MRI revealed a long-segment T2 hyperintense intramedullary lesion opposite to the C3 to D4 vertebral levels with mild heterogeneous enhancement on the post contrast study [Figure 1].
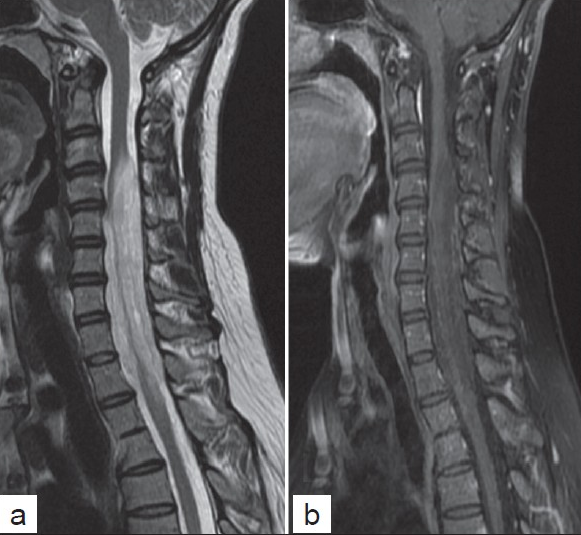
- (a) Sagittal T2 and (b) post contrast T1-weighted MR images showing a long segment T2 hyperintense and mild hetrogenous enhancing lesions in the cervical cord, showing cord expansion.
She underwent subtotal excision of the lesion with cervical laminoplasty. Intraoperatively, the lesion was firm, with a poor plane of demarcation from the normal cord, and was seen intermingling with posterior and anterior nerve rootlets. It was eccentric in location, involving more of the left hemicord, with exophytic components [Figure 2].
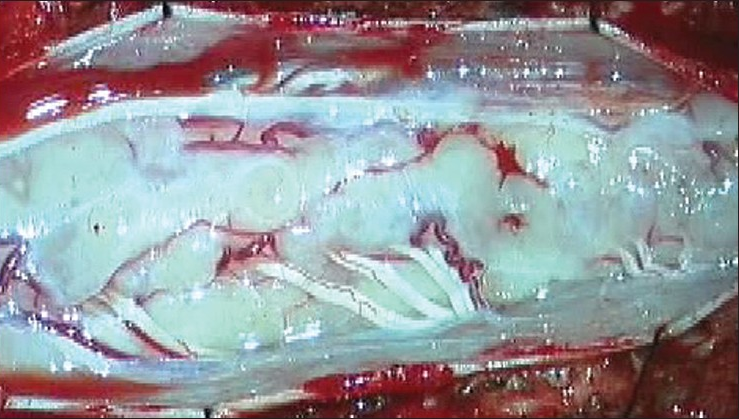
- Surgical finding: Typical exophytic subpial lesions, with lesion on either sides of the dorsal nerve roots.
All of the resected tissue submitted for histopathological analysis was processed and embedded in three paraffin blocks. Histopathological examination [Figure 3] revealed characteristic features of a subependymoma (WHO grade 1) with small monomorphic cells arranged in a lobular pattern against a finely fibrillary background, with occasional rosette like pattern. Features of pleomorphism, mitotic activity, endothelial cell proliferation, and necrosis were not seen. The MIB-1 proliferative index was low (<1% of tumor cells)(). Immunohistochemistry revealed diffuse positivity for Glial Fibrillary Acidic Protein (GFAP), whereas tumor cells failed to express epithelial membrane antigen (EMA).

- Histopathology: (a) H and E staining shows lobular pattern arrangement of monomorphic nuclei in fibrillary pattern (×400). (b) MIB-1 labeling index is less than 1% of tumor cells (not shown in figure) (×400). Immunohistochemistry for (c) GFAP highlights diffusely positive tumor cells in a fibrillary background (×400) (d) Tumor cells are negative for EMA (×400)
She did not receive any adjuvant treatment and has been on follow up for 6 months with periodic MR imaging. She does not report any progression of symptoms.
Discussion
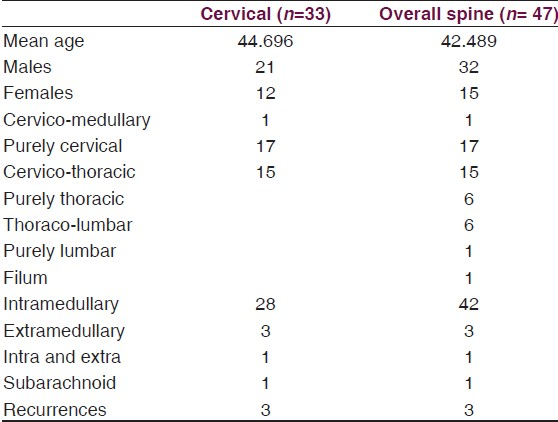
Subependymomas are rare central nervous system tumors accounting for 0.7% of all intracranial neoplasms and 8.3% of all ependymal tumors. They are located most frequently in the fourth ventricle (50–60% of cases) followed by the lateral ventricles (30–40%). So far, only 47 cases have been reported in the English literature in the spinal cord; of which 32 cases were located in the cervical, cervico-medullary, and cervico - thoracic locations. [Table 1]. Most are intramedullary in location, with few subarachnoid and extramedullary variants mentioned in the literature. The symptomatic subependymoma generally occur after 40 years of age and are rare in childhood.[1–3]
They have a mean age of presentation of about 44 years with a strong male preponderance [Table 2].
Histopathologically these tumors are low grade (WHO grade 1), characterized by homogenous cells with sparse cellularity showing clustering of nuclei over a fibrillary background formed by cell processes and occasional occurrence of microcysts. The tendency to form pseudorosettes and the cells being round with hyperchromatic nuclei are reminiscent of ependymomas.[1256]
Immunohistochemical characteristics are however identical to that of astrocytic neoplasms with diffuse GFAP and S-100 protein positivity. In contrast to ependymomas, subependymomas are negative for EMA. Ultrastructurally, they demonstrate both astrocytic and ependymal characteristics. The former is evidenced by the processes containing intermediate filaments and the latter by microlumens, cilia, microvilli, and intercellular junctions.[1256]
Histogenesis of Spinal Subependymoma
The histogenesis of these tumors has been much debated. Due to their ultrastructural similarities with ependymal cells, they were initially thought to arise from ependymal cells, with reactive astrocytic proliferation. Since immunohistochemical profile favors a glial origin, they were grouped with low-grade glial tumors. Tanycytes were also proposed as source of origin, due to the presence of ultrastructural features of glial as well as ependymal components. However, the presence of round cells rather than spindle-shaped cells differentiates these lesions from tanycytic ependymomas. The identification of cells similar to subependymal glial precursor cells suggested alternate cell of origin.[1256]
Subependymal zone glial precursor cell origin cannot explain the predominant peripheral and exophytic location of these lesions in spinal cord unlike their intracranial counterparts. The current concept seems to suggest that these progenitor cells exist throughout the spinal cord white matter as well as the root entry zones. They are more localized in the outer subpial white matter than the medial zones, especially at the dorsal and ventral spinocerebellar tracts. They tend to proliferate within their template zones and do not possess trans-zonal migratory ability. The tumor origin from these cells better explains the eccentric, subpial, and exophytic locations of spinal subependymomas.[27]
The progenitor cells origin postulated by Horner et al is an alternative to the conventional old postnatal theory [Type 1, Figure 4] where the progenitor cells migrate from the subependymal zone to the outer zones. There are two alternative postulates: one where the stem cell divides asymmetrically and the daughter cell migrates to the outer white matter where it remains as a glial progenitor cell and multiplies [Type 2, Figure 4], and the second which postulates that the stem cells exist separately in the outer zone where tracts are constantly proliferating even postnatally and these give rise progenitor glial cells [Type 3, Figure 4].[7]
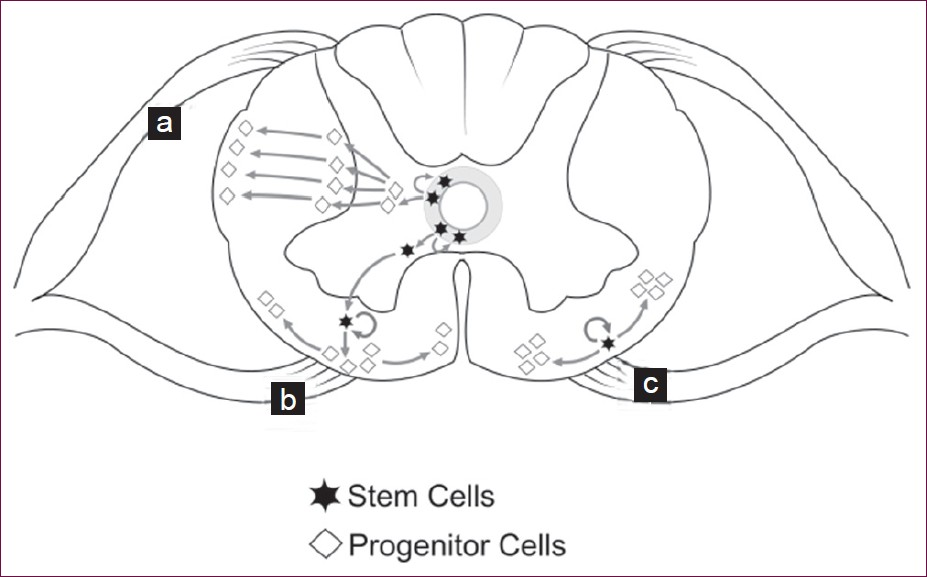
- Progenitor glial cell origin theories. (a) Post natal theory: where the subependymal stem cells give rise to glial progenitor cells which then migrate to the periphery. (b and c) Alternate new theories where the stem cells themselves migrate to the periphery and become glial progenitor cells (b) or the stem cells already existing in the periphery of the cord become glial progenitor cells (c).
Irrespective of their origin the subpial spinal white matter progenitor cells may be the origin of the spinal subependymoma.
The subependymomas reported are more commonly located in the cervical cord and the cervico-thoracic cord followed by the pure thoracic and lumbar segments. One rare case occurring in the filum teminale has also been reported [Table 2]. Radiologically, these lesions are located eccentrically within the cord, with iso or hypointense on T1-weighted images and mild hyperintensity on T2-weighted images. There is minimal diffuse or no enhancement on contrast.[1–48]
These lesions can usually be excised totally. Only three cases have been reported with recurrence of the tumor after initial total excision. All of them had a resurgery and were not subjected to adjuvant radiation or chemotherapy despite recurrence. The chances of recurrence after total excision are low; hence, a total excision should always be attempted [Tables 1 and 2].
However, as with our case, the size of the lesion and its extensive intermingling with the nerve roots made it sometimes necessary to settle for a subtotal excision. In such cases too, a favorable outcome has been reported without adjuvant therapy. There have been instances of postoperative radiation following subtotal excision, but it is generally not recommended. Instead a periodic follow up would be in order. Residual or recurrent tumor on follow up after gross total resection should be strongly considered for a re-excision rather than adjuvant therapy.[2–68]
Source of Support: Nil
Conflict of Interest: None declared
References
- Intramedullary subependymoma of the spinal cord: A case report and review of literature. Clin Neurol Neurosurg. 2003;106:63-8.
- [Google Scholar]
- Intramedullary subependymoma occupying the right half of the thoracic spinal cord-case report. Neurol Med Chir (Tokyo). 2002;42:349-53.
- [Google Scholar]
- Intramedullary subependymoma of the thoracic spinal cord. J Clin Neurosci. 2009;16:851-3.
- [Google Scholar]
- Imaging appearance of subependymoma: A rare tumor of the cord. Indian J Cancer. 2008;45:33-5.
- [Google Scholar]
- Atypical subependymoma of the spinal cord: Ultrastructural and immunohistochemical studies. Neurosurgery. 1990;27:319-25.
- [Google Scholar]
- Subependymoma of the spinal cord and review of the literature. Pathol Int. 2003;53:169-73.
- [Google Scholar]
- Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218-28.
- [Google Scholar]
- Subependymoma of the cervical spinal cord.A case report of long-term survival. Minn Med. 1991;74:21-4.
- [Google Scholar]
- Subependymoma of the spinal cord.Case report and review of the literature. Neurosurg Rev. 1992;15:65-9.
- [Google Scholar]
- Intramedullary subependymoma of the cervical spinal cord: A case report with immunohistochemical study. Int J Neurosci. 2010;120:676-9.
- [Google Scholar]






