Translate this page into:
Burr-Hole Evacuation of Chronic Subdural Hematoma: Biophysically and Evidence-Based Technique Improvement
Address for correspondence: Dr. Martin Májovský, Department of Neurosurgery and Neurooncology, First Faculty of Medicine of Charles University, Military University Hospital, U Vojenske Nemocnice 1200, Prague 6, Czech Republic. E-mail: martin.majovsky@uvn.cz
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Chronic subdural hematoma (CSDH) is one of the most common neurosurgical conditions. Despite ongoing efforts, recurrence and reoperation rates after surgical treatment remain high. We synthesize scientific evidence on the treatment of CSDH with biophysical principles and then propose a simple and effective surgical technique aiming to reduce the recurrence rate. Under local anesthesia, one burr hole is placed in the area above the maximum hematoma thickness. One drain is inserted into the dorsal direction to the deepest point of the hematoma cavity, and a second drain is inserted frontally into the highest point. Next, saline is gently instilled to the dorsal drain to eliminate air from the hematoma cavity through the frontal drain. Once saline has filled the frontal drain, the frontal drain is removed. The dorsal drain is left in situ for 48 h, and the pressure within the cavity may be adapted hydrostatically. We implemented evidence-based conclusions of previous studies and modified the classical burr-hole technique to reduce the recurrence rate. As a result, we developed a straightforward surgical procedure that is possible to perform under local anesthesia, suitable for everyday practice in rural and remote areas while working with limited resources. The novelty of this technique is in the purposeful reduction of postoperative pneumocephalus, a known independent factor of recurrence. Subdural air is eliminated during surgery using a two-drain system. Safety and efficacy of the technique need to be evaluated in future clinical trials.
Keywords
Chronic subdural hematoma
evidence-based medicine
head trauma
neurosurgery
pneumocephalus
INTRODUCTION
Chronic subdural hematoma (CSDH) is among the most common neurosurgical conditions. Although CSDH is usually not a life-threatening condition, its clinical course is not benign.[1] Perioperative morbidity ranges from 0% to 25% and mortality from 0% to 32%.[23] The generally accepted mortality rate is usually 8%. Furthermore, even after a successful evacuation of CSDH, the excess mortality rate can be seen up to 1 year after surgery.[4]
Three principal surgical procedures are commonly used as follows: twist-drill craniostomy, burr hole, and craniotomy. Many modifications of these procedures have been described; however, none have solved the main problem of CSDH surgery, namely a high recurrence rate. Recurrence of CSDH is usually defined as reaccumulation of hematoma fluid that needs reoperation; defined in this way, the recurrence rate ranges from 0.4% to 33.3%.[5] In most studies, the recurrence rate is around 10%.
The literature offers evidence on the effectiveness of certain surgical nuances. Systematic reviews have shown the superiority of irrigation and placing a drain in the hematoma cavity to decrease the recurrence rate.[678] Another independent factor of recurrence that might be impacted during surgery is the amount of air that enters the hematoma cavity (i.e., pneumocephalus).[910111213] Reduction of pneumocephalus is often disregarded during surgery, and most surgical techniques do not address this issue at all.
This paper aimed to synthesize scientific evidence on the treatment of CSDH with biophysical principles and to propose a simple and effective surgical technique, suitable for everyday practice in rural and remote areas while working with limited resources.
TECHNIQUE DESCRIPTION
The surgery is generally performed under local anesthesia and is usually well tolerated. The patient's head is rotated approximately 45° away from the side of the hematoma and fixed by a tape in a horseshoe headrest. One burr hole is made in the area above the maximum hematoma thickness. In the event of a large holohemispheric CSDH, the burr hole is placed approximately at the coronal suture to avoid potential motor cortex injury. One drain is inserted into the dorsal direction to the deepest point of the hematoma cavity, and the other one is inserted frontally into the highest point [Figure 1a]. The exact distance from the burr hole to the deepest point and to the highest point is measured by computed tomography (CT) scan before surgery, and this distance is marked on each drain.
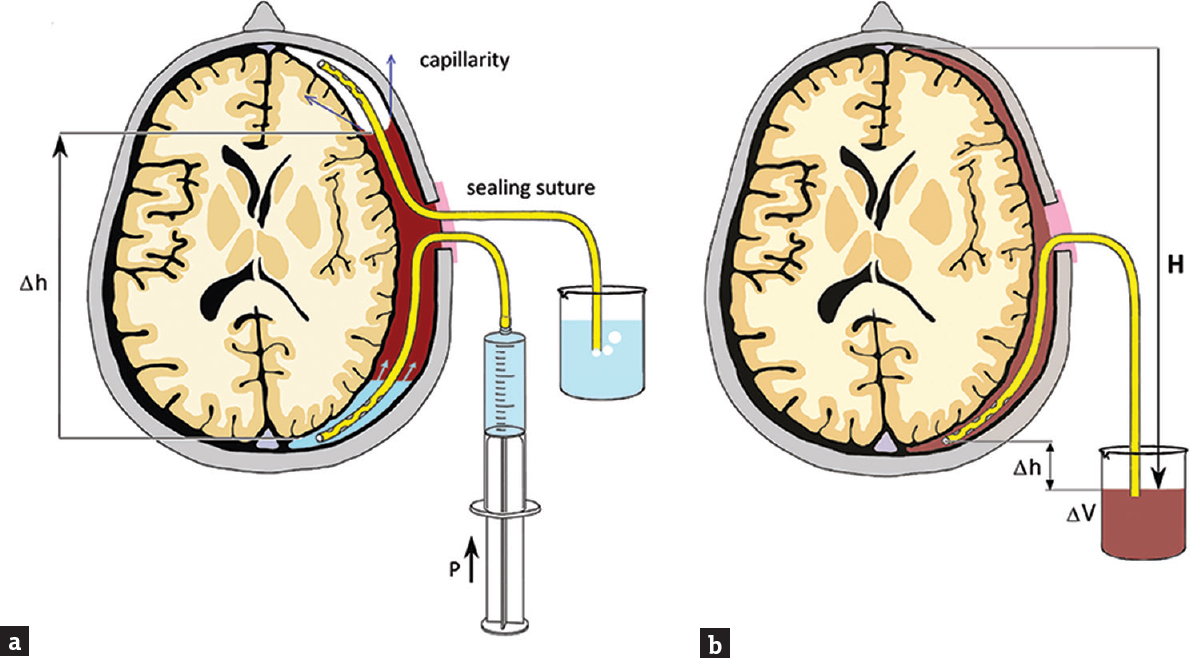
- (a) Schematic depiction of intraoperative saline irrigation using two drains (frontal drain and dorsal drain). Note the tip of the frontal drain in the air bubble trapped in the subdural cavity. The air is eliminated from the subdural cavity through the frontal catheter. Δh shows the height difference attributed to hydrostatic pressure. (b) Postoperative situation showing no subdural air. Only the dorsal drain is left in place to drain the residual fluid under the hydrostatic pressure (ρgH). The outflow ΔV (and indirectly the intracavity pressure) may be controlled by the hydrostatic levels of the internal and external ends of the drain (Δh)
The hematoma cavity is irrigated copiously until the drainage fluid runs clear. Thereafter, the wound is closed in a two-layer fashion to prevent any hematoma-atmosphere communication. Then, the saline is gently instilated to the dorsal drain to eliminate air from the hematoma cavity by the way of the frontal drain [Figure 2]. When saline fills the frontal drain, the frontal drain is removed and air bubbles, if present, stop to appear in the effluate [Figure 1b]. The patient is left on strict bed rest for 2 days. The hydrostatic level between the intracranial and extracranial ends of the dorsal drain (hence the pressure within the cavity) may be set according to the volume and composition of the effluate. A CT scan is performed on the 2nd postoperative day, and the dorsal drain is removed [Figure 3].
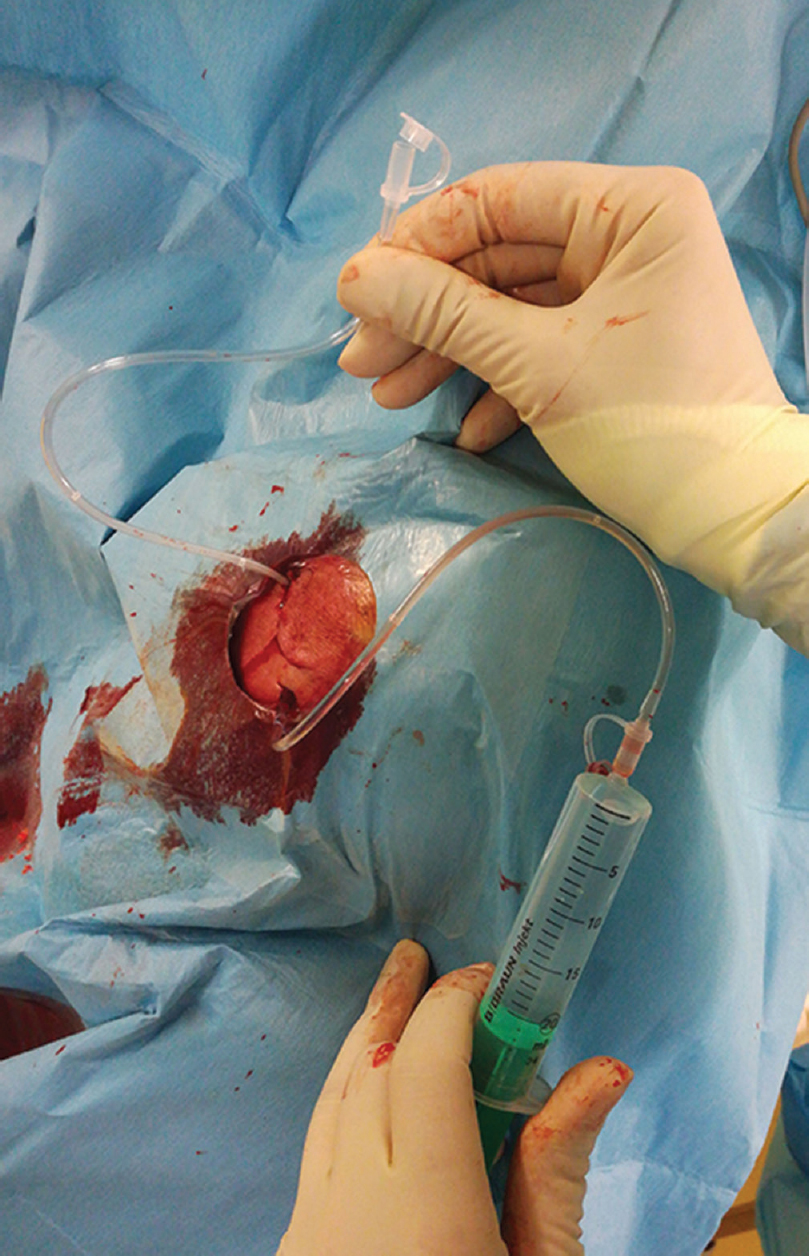
- Intraoperative photo after wound suturing showing two drains (frontal drain and dorsal drain). Analogous situation is depicted in Figure 1a
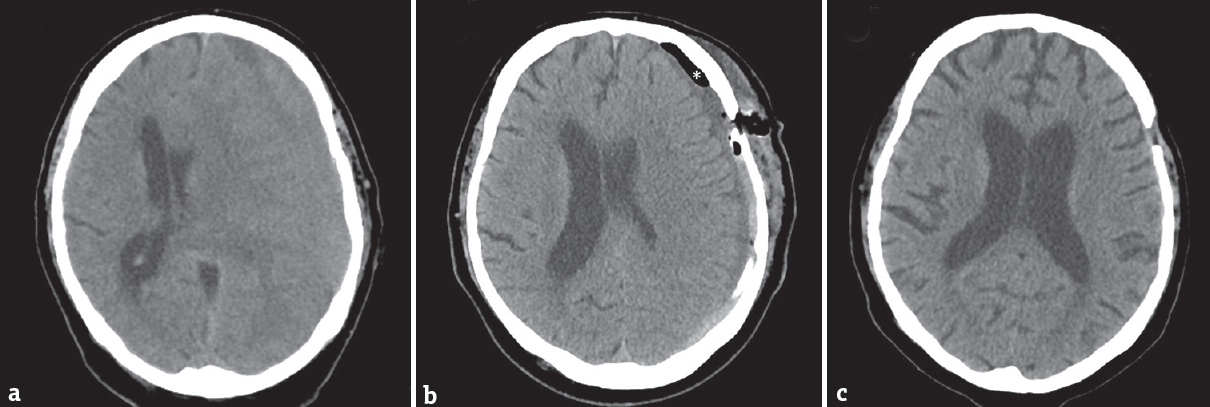
- Computed tomography scan of the patient with chronic subdural hematoma before surgery (a). Early postoperative computed tomography (b) showing minimal residual subdural air (asterisk). After 1 month, complete resolution of chronic subdural hematoma and subdural air (c)
So far, the proposed technique was used in 18 patients with good postoperative outcome and no recurrence. About 56% of the patients were on antiplatelet medication and 28% on oral anticoagulants preoperatively. The most common symptom was hemiparesis (78%), followed by a decreased level of consciousness (22%), headache (17%), and aphasia (6%). The mean maximum hematoma thickness was 23.0 mm ± 5.3 mm; mean operative time was 36 ± 8 min; and the mean postoperative pneumocephalus volume was 3.8 ± 2.1 ml on CT scan performed on the 2nd postoperative day. The mean postoperative follow-up was 9.3 months ± 3.6 months. No reoperation was performed, and no complication occurred in these patients during the follow-up. Modified Rankin Scale (mRS) measured at the follow-up period was 0 in 16 cases, 1 in one case, and 2 in one case.
DISCUSSION
How to reduce the recurrence rate?
Despite efforts, the recurrence rate in surgery for CSDH remains high. When defined as symptomatic reaccumulation of hematoma fluid that needs reoperation, the recurrence rate ranges (widely) from 0.4% to 33.3%.[5] Whereas some factors of recurrence cannot be influenced (age, comorbidities, severe neurological deficit, volume and internal structure of hematoma, and bilaterality), others can.
Factors of recurrence that can be influenced are mainly certain operative nuances and postoperative measures such as the type of trepanation, intraoperative irrigation, drain insertion and position, air that enters the subdural space, and postoperative patient position. Procedures available for CSDH patients include twist-drill craniostomy, burr hole, and craniotomy. A burr hole seems to have the best cure-to-complication ratio when compared with the twist-drill craniostomy and craniotomy,[237814] which was confirmed by Monte Carlo simulation techniques and decision analytical methods.[15] Evidence favors intraoperative irrigation of sterile saline to wash out the hematoma, despite potential risks associated with the introduction of air and infection.[161718] Meta-analyses show that inserting a drain into the hematoma cavity and leaving it in place for 24–48 h to drain residual hematoma fluid is beneficial in reducing recurrence without increasing risk of acute bleeding or infection.[678] Santarius et al. performed a randomized trial that demonstrated not only a decrease in recurrence when using the drain but also a decrease at a 6-month mortality rate.[19] Leaving postoperatively two drains instead of one was studied by Gernsback et al. and showed no additional benefit.[20] Another important independent factor of recurrence is postoperative pneumocephalus, i.e., the amount of air that enters the hematoma cavity during surgery.[910111213] Only a few papers, which were mainly technique descriptions, have examined reducing postoperative pneumocephalus. We discuss this issue more in depth in a separate paragraph (see below). The optimal postoperative posture of the patient was examined in only a few studies, with results indicating no meaningful differences between sitting and supine postoperative positions regarding the recurrence rate.[212223] Concerning our technique, we assume that the supine position and bed rest for 48-postoperative hours are needed to ensure hydrostatic pressure gradients as described above.
When developing our technique, we included all the above-mentioned measures that are supported by the scientific evidence.
How to reduce postoperative pneumocephalus?
The most important technique is to position patient's head on a horseshoe headrest with burr hole at the highest point if possible. Several other techniques have been proposed to reduce postoperative pneumocephalus, with some being quite complicated and invasive. Pioneers in this research are mostly Japanese investigators. Intraoperative intrathecal injection of saline for cerebral reexpansion was described in two papers.[2425] The authors used Ringer's solution and Ringer's lactate solution that were introduced through a lumbar needle to the subarachnoid space until the brain reexpanded to the inner table of the skull and thus subdural air eliminated. In the study by Grisoli et al., the amount of fluid ranged from 40 to 300 ml.[24]
Another approach to avoid air collection in the hematoma cavity is replacing air by another gas that is absorbed into the circulation faster than air. Kitakami et al. used carbon dioxide to fill the hematoma cavity after blood evacuation.[26] Carbon dioxide is absorbed 250 times faster than air, which is one of the reasons for its use in abdominal surgery to create pneumoperitoneum during laparoscopy. The authors observed almost complete absorption 24 h after surgery and the recurrence rate was 5.2%. Similar techniques using oxygen instead of carbon dioxide have been published by Aoki et al. and Takeda et al.[2728]
The direction of drain placement to reduce pneumocephalus has been examined in only a few studies. Nakaguchi et al., for instance, found a significant reduction in both postoperative pneumocephalus and in the recurrence rate in patients with the frontal direction of the drain when compared with patients with a drain placed parietally and occipitally.[12] The authors explained their results by easier evacuation of the air collection by a frontally placed drain when patients were lying in a supine position. Shiomi et al. observed a significantly longer time to recurrence in patients with frontal drain direction and a trend toward a lower recurrence rate.[10] On the other hand, Ohba et al. did not find a statistically significant difference between the frontal and dorsal position of the drain.[9]
A conservative measure to resolve postoperative pneumocephalus is administration of supplemental oxygen. The rationale for this approach is that an increase in FiO2 decreases the concentration of nitrogen within the blood and brain tissue and thereby increases the nitrogen concentration gradient for absorption. Gore et al. performed a randomized trial on 13 patients that underwent elective craniotomy (non-CSDH patients) with a mean initial air volume of 50 ml.[29] The results showed that 24-h administration of 100% normobaric oxygen significantly increased the absorption rate of postcraniotomy pneumocephalus as compared with breathing room air. The potential use of this treatment in CSDH patients is limited because the usual volume of air is higher than 50 ml, and longer administration of concentrated oxygen is linked to the risk of pulmonary toxicity.
Advantages of the present technique
Our technique combines the aforementioned scientific evidence (i.e., intraoperative irrigation, reduction of pneumocephalus, and one drain for 48-h placement) with basic physical principles. In our technique, postoperative subdural air accumulation is avoided by replacing it with saline using two drains during surgery. The air is pushed upward by the ascending level [Δh in Figure 1] of the inflowing fluid. The superficial tension at the air-fluid interface facilitates the coalescence of bubbles into one that is finally expelled by the frontal drain. When air is eliminated, the frontal drain is removed in the operating room, and the dorsal drain is left in place for 48-h postsurgery. We leave in place dorsal drain despite the fact, that some studies showed better outcome in frontally placed drain. The superiority of the frontal drain placement was explained in the studies by easier air elimination when the patient is postoperatively in the supine position allowing the air to migrate passively frontally and to form a bubble around the tip of the frontal drain. In our technique, this process is active due to pressure [P in Figure 1] and is thus accelerated. Therefore, the use of the frontal drain is not necessary. On the contrary, the remaining dorsal drain improves the removal of the residual fluid thanks to hydrostatic pressure gradient setup [H in Figure 2]. The outflow which facilitates expansion of the brain tissue may be controlled by the level between the intracranial and extracranial ends of the drain [Δh in Figure 2].
Cecchini described a similar technique to ours with one important difference. The drain that is left in place is introduced through the parietal burr hole and faces frontally.[30] The tip of the drain is at the highest point of the CSDH cavity when the patient is in the supine position. Therefore, this setting does not benefit from the maximum hydrostatic pressure as compared with our technique see Figure 1a. In addition, we consider the placing of the burr hole at the parietal eminence as more difficult to perform under local anesthesia and riskier regarding potential injury of the motor cortex. A similar technique was described by Weigel et al.,[31] however, these authors left the postoperatively frontal drain in place, which we consider less effective.
Limitations
This paper has several limitations that need to be addressed. First, it is only a technique description, and therefore, clinical trials are required to clarify its efficacy. We consider the present development of technique as Stage 2a according to IDEAL framework,[32] and next stages are going to begin. Second, the technique demands the specific placement of subdural drains, which might be challenging. During insertion of the drain, it may move to an undesired direction even without the neurosurgeon's awareness. Proper positioning of the drain might be checked by an endoscope; however, it requires additional equipment.[33] Third, placing two drains instead of the usual one, even temporarily, might potentially increase the risk of bridging vein and brain surface damage. Meta-analyses did not show higher complication rate associated with single drain use.[67] Therefore, we speculate that two drains use do not increase complication rate either. Fourth, the presented technique is the best suitable for homogenous hematomas without compartmentalization. Hematoma with at least two compartments needs to be evacuated with membrane opening. Furthermore, we do not use the presented technique for hematomas with acute component because complete irrigation is not possible.
CONCLUSION
A high recurrence rate is a major concern of CSDH surgery. We implemented evidence-based conclusions of previous studies to reduce recurrence while keeping the surgical procedure straightforward and feasible to perform under a local anesthesia. The novelty of the present technique consists of the purposeful reduction of postoperative pneumocephalus, which has been shown to be an independent factor of recurrence. Subdural air is eliminated during surgery using a two-drain system (frontal drain and dorsal drain). The frontal drain is removed at the end of the surgical procedure while the dorsal drain is left in situ for 48 h to allow the evacuation of residual hematoma fluid. Obviously, the future clinical trials are needed to establish the safety and efficacy of the technique.
Financial support and sponsorship
This study was supported by grant no. MO 1012NK funded by the Ministry of Defense of the Czech Republic and grant no. Q25/LF1/2 funded by the Charles University.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Chronic subdural hematoma in elderly patients: Is this disease benign? Neurol Med Chir (Tokyo). 2017;57:402-9.
- [Google Scholar]
- The surgical management of chronic subdural hematoma. Neurosurg Rev. 2012;35:155-69.
- [Google Scholar]
- Outcome of contemporary surgery for chronic subdural haematoma: Evidence based review. J Neurol Neurosurg Psychiatry. 2003;74:937-43.
- [Google Scholar]
- Chronic subdural hematoma in the elderly: Not a benign disease. J Neurosurg. 2011;114:72-6.
- [Google Scholar]
- Surgical treatments for chronic subdural hematomas: A comprehensive systematic review. World Neurosurg. 2016;86:399-418.
- [Google Scholar]
- External drains versus no drains after burr-hole evacuation for the treatment of chronic subdural haematoma in adults. Cochrane Database Syst Rev. 2016;8:CD011402.
- [Google Scholar]
- Chronic subdural hematoma management: A systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014;259:449-57.
- [Google Scholar]
- Chronic subdural hematoma: A systematic review and meta-analysis of surgical procedures. J Neurosurg. 2014;121:665-73.
- [Google Scholar]
- The risk factors for recurrence of chronic subdural hematoma. Neurosurg Rev. 2013;36:145-9.
- [Google Scholar]
- Relationship of direction of drainage tube and recurrence in chronic subdural hematoma. No Shinkei Geka. 2002;30:823-7.
- [Google Scholar]
- Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001;41:371-81.
- [Google Scholar]
- Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93:791-5.
- [Google Scholar]
- Clinical factors of recurrent chronic subdural hematoma. Neurol Med Chir (Tokyo). 2001;41:382-6.
- [Google Scholar]
- Chronic subdural haematoma: Modern management and emerging therapies. Nat Rev Neurol. 2014;10:570-8.
- [Google Scholar]
- Choosing the best operation for chronic subdural hematoma: A decision analysis. J Neurosurg. 2010;113:615-21.
- [Google Scholar]
- Factors predicting recurrence of chronic subdural haematoma: The influence of intraoperative irrigation and low-molecular-weight heparin thromboprophylaxis. Acta Neurochir (Wien). 2012;154:1063-7.
- [Google Scholar]
- A comparative study of treatments for chronic subdural hematoma: Burr hole drainage versus burr hole drainage with irrigation. Kurume Med J. 2011;58:35-9.
- [Google Scholar]
- Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien). 2001;143:1041-4.
- [Google Scholar]
- Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: A randomised controlled trial. Lancet. 2009;374:1067-73.
- [Google Scholar]
- To drain or two drains: Recurrences in chronic subdural hematomas. World Neurosurg. 2016;95:447-50.
- [Google Scholar]
- The management and outcome for patients with chronic subdural hematoma: A prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg. 2017;127:732-9.
- [Google Scholar]
- The role of postoperative patient posture in the recurrence of chronic subdural hematoma: A prospective randomized trial. Surg Neurol. 2002;58:385-7.
- [Google Scholar]
- The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007;61:794-7.
- [Google Scholar]
- Perioperative lumbar injection of Ringer's lactate solution in chronic subdural hematomas: A series of 100 cases. Neurosurgery. 1988;23:616-21.
- [Google Scholar]
- Intraoperative lumbar injection of Ringer's solution for surgical treatment of chronic subdural hematomas. No Shinkei Geka. 1991;19:511-6.
- [Google Scholar]
- Carbon dioxide gas replacement of chronic subdural hematoma using single burr-hole irrigation. Surg Neurol. 1995;43:574-7.
- [Google Scholar]
- A new simple therapeutic method for chronic subdural hematoma without irrigation and drainage. Acta Neurochir (Wien). 2006;148:541-6.
- [Google Scholar]
- A new therapeutic method for chronic subdural hematoma in adults: Replacement of the hematoma with oxygen via percutaneous subdural tapping. Surg Neurol. 1992;38:253-6.
- [Google Scholar]
- Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg. 2008;108:926-9.
- [Google Scholar]
- Avoiding pneumocephalus after chronic subdural hematoma evacuation: The temporary double drainage technique. 2015. Neurol Disord. 1:113-5. Available from: Available from: http://www.esciencecentral.org/journals/neurological-disorders-abstract.php?abstract_id=63448
- [Google Scholar]
- Treatment concept of chronic subdural haematoma according to an algorithm using evidence-based medicine-derived key factors: A prospective controlled study. Br J Neurosurg. 2015;29:538-43.
- [Google Scholar]
- IDEAL framework for surgical innovation 1: The idea and development stages. BMJ. 2013;346:f3012.
- [Google Scholar]
- Flexible endoscope-assisted evacuation of chronic subdural hematomas. Acta Neurochir (Wien). 2016;158:1987-92.
- [Google Scholar]






