Translate this page into:
Botulinum Toxin for Refractory Trigeminal Neuralgia: A Trial Sequential Analysis of Randomized Clinical Trials
Address for correspondence: Dr. Kannan Sridharan, Associate Professor, Department of Pharmacology and Therapeutics, College of Medicine and Medical Sciences, Arabian Gulf University, Manama, Bahrain. E-mail: skannandr@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
In this issue, Caldera et al.[1] published a study where the authors observed a significant reduction in the symptoms of trigeminal neuralgia (TN) following injection of botulinum toxin at the trigger point in a cohort of South Asian patients. In our earlier network meta-analysis of interventions for refractory TN, we also observed that botulinum toxin injection was associated with significant benefits compared to placebo.[2] A previous systematic review assessing the prospective studies on botulinum toxin in TN observed therapeutic response ranging between 70% and 100% without any significant major adverse events.[3] Although botulinum toxin is approved only for treating chronic migraine, the analgesic effects of botulinum toxin in TN have already been reported first in 1998. Since then, numerous nonrandomized and observational studies and four randomized controlled clinical trials[4567] have been conducted with botulinum toxin in refractory TN. We carried out a trial sequential analysis[8] assessing the efficacy of botulinum toxin from the estimates of three randomized clinical trials[456] with the methodology described similarly elsewhere.[7] Relative risk (95% confidence intervals) of patients with pain relief was the outcome variable, and one study[7] did not report this outcome. We observed statistically significant pooled estimates (2.86 [1.82, 4.48]) favoring botulinum toxin [Figure 1], and the trial sequential analysis confirmed the existence of adequate evidence for therapeutic utility of botulinum toxin. Although there is no expert consensus on using botulinum toxin in refractory TN due to lack of robust and long-term follow-up studies and cost-effectiveness data, the agent looks promising to use based on trial sequential analysis principles.
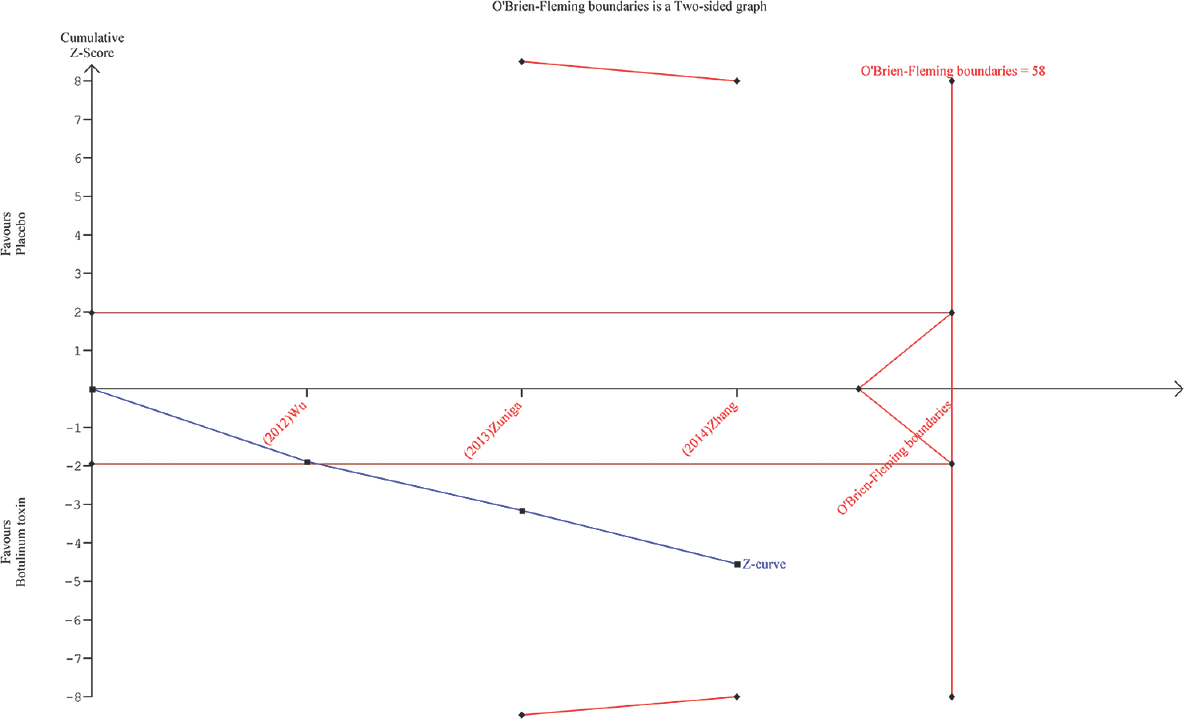
- Trial sequential analysis of botulinum toxin compared to placebo for number of patients with pain relief. Blue line indicates the trend in the pooled estimates with addition of results from each trial, and the final pooled estimates were observed to favor botulinum toxin
REFERENCES
- Efficacy of botulinum toxin type A in trigeminal neuralgia in a South Asian Cohort. J Neurosci Rural Pract. 2018;9:100-5.
- [Google Scholar]
- Interventions for refractory trigeminal neuralgia: A bayesian mixed treatment comparison network meta-analysis of randomized controlled clinical trials. Clin Drug Investig 2017 doi:10.1007/s40261-017-0553-9
- [Google Scholar]
- Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: A systematic review. J Headache Pain. 2013;14:72.
- [Google Scholar]
- Botulinum toxin type A for the treatment of trigeminal neuralgia: Results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 2012;32:443-50.
- [Google Scholar]
- Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J Headache Pain. 2014;15:65.
- [Google Scholar]
- Acute treatment of trigeminal neuralgia with onabotulinum toxin A. Clin Neuropharmacol. 2013;36:146-50.
- [Google Scholar]
- Botulinum toxin-type A: Could it be an effective treatment option in intractable trigeminal neuralgia? J Headache Pain. 2013;14:92.
- [Google Scholar]
- Vasoactive agents for hepatorenal syndrome: A mixed treatment comparison network meta-analysis and trial sequential analysis of randomized clinical trials. J Gen Intern Med 2017 doi:10.1007/s11606-017-4178-8
- [Google Scholar]





