Translate this page into:
Blooming in the Hypothalamus
Address for correspondence: Dr. Santosh P. V. Rai, Department of Radiodiagnosis, Kasturba Medical College, Manipal Academy of Higher Education, Lighthouse Hill Road, Mangalore - 575 001, Karnataka, India. E-mail: santosh.rai@manipal.edu
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cavernomas are vascular malformations which are collections of endothelium-lined sinusoids without intervening cerebral parenchyma. Hypothalamic location of cavernoma is extremely rare. We present a case of a 34-year-old male who presented with complaints of recent memory loss and vomiting. On magnetic resonance imaging with gradient sequences and contrast, a diagnosis of hypothalamic cavernoma was suggested. Excision of lesion was performed by a right parasagittal pericoronal craniotomy via transcallosal approach. Intraoperative findings and histopathology examination corroborated the diagnosis. The uniqueness of this case report is in the susceptibility-weighted sequence which led to the radiological diagnosis.
Keywords
Cavernous hemangioma
cavernous malformation
hypothalamic
INTRODUCTION
Cavernomas are vascular malformations which are collections of endotheliumlined sinusoids without intervening cerebral parenchyma. Hypothalamic location of cavernoma is extremely rare.[1]
CASE REPORT
A 34-year-old male patient, a known hypertensive on treatment, presented with a history of memory loss of 4 months’ duration which got aggravated since the last 1 month. He also had vomiting for the past 1 month. There was no history of seizures or focal neurological deficit. Clinical neurological examination was unremarkable and vitals were stable. Glasgow Coma scale was 15/15. Cerebellar signs were absent. Magnetic resonance imaging (MRI) revealed a suprasellar lesion which was T1 hyperintense [Figure 1a and b] and T2 hyperintense, with hypointense rim involving bilateral hypothalamus [Figure 1c]. The lesion was not showing any significant change in the postcontrast T1 [Figure 1d] and patchy diffusion restriction [Figure 1e]. Anteriorly, the lesion was abutting the anterior commissure and lamina terminalis, anteroinferiorly the optic chiasm, inferiorly the optic tracts, and posteroinferiorly the mammillary bodies and part of the anterior midbrain. The lesion was impinging on the left foramen of Munro and there was mild dilatation of anterior horn of the left lateral ventricle. On the susceptibility imaging sequences, blooming was seen within the lesion on magnitude, phase, and fusion images [Figure 1f-h] The pituitary gland was normal. These MRI findings suggested an hemorrhagic lesion in the region of hypothalamus with hypointense rim and no significant contrast enhancement pointing toward a diagnosis of hypothalamic cavernoma. However, differentials considered were other hemorrhagic lesions in the region of hypothalamus (hemorrhagic neoplasms, glioma), lesions containing lipid (hypothalamic hamartoma), lesions containing protein (colloid cyst), lesions with calcifications (craniopharyngioma), and other lesions such as germinoma. Excision of the lesion was performed by a right parasagittal pericoronal craniotomy via transcallosal approach. Intraoperatively, this lesion had a typical mulberry-like appearance due to dilated, thin-walled vascular channels [Figure 2]. On histopathology, there were cavernous spaces with a thin sclerotic wall, few of which were filled with blood and the absence of intervening glial tissue was characteristic of a cavernoma [Figure 3].

- Magnetic resonance imaging showing lesion to be hyperintense on the sagittal T1-weighted (a) and axial T1-weighted images (b), hyperintense with hypointense rim on the axial T2 (c), not showing any significant signal change in the postcontrast axial T1 (d), and showing patchy diffusion restriction (e), and significant blooming on the magnitude (f), phase (g), and fusion (h) images on the susceptibility imaging sequences
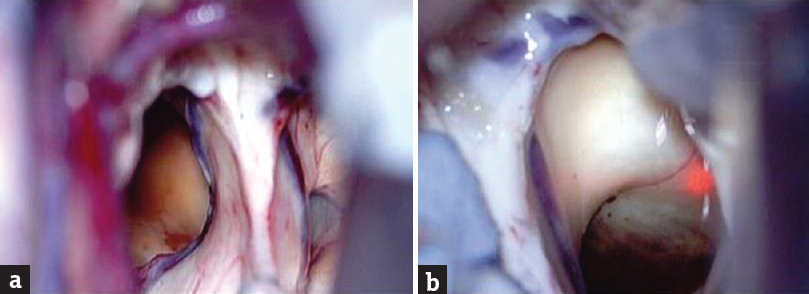
- Intraoperative photograph showing right parasagittal pericoronal craniotomy via transcallosal approach (a) Intraoperatively, this lesion had a typical mulberrylike appearance (b) due to dilated, thinwalled vascular channels
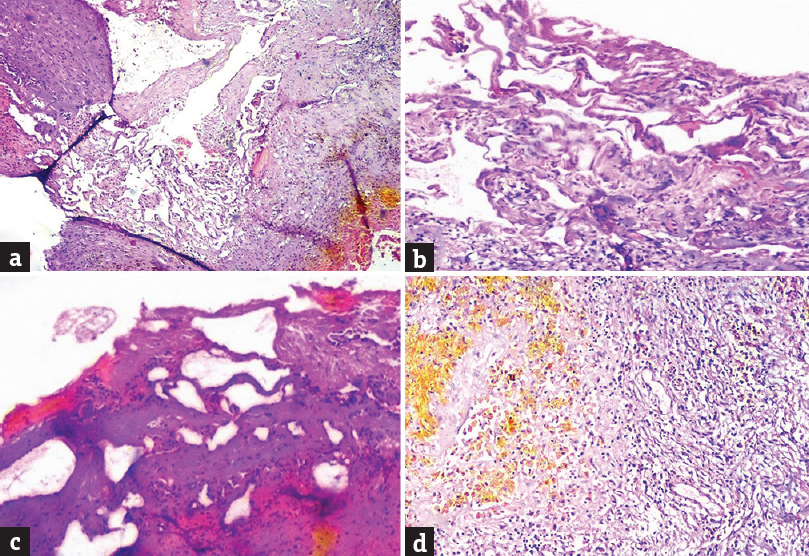
- (a and b) Small and large cavernous spaces having sclerotic wall, (c) cautery artifact, (d) yellow-brown hemosiderin pigment indicative of past hemorrhage
DISCUSSION
Cavernomas are rare, occurring in 0.3%–0.7% of the general population. Hypothalamic location of cavernoma is even rarer accounting for only 1% or less of the cavernomas.[12] Based on the flow within the lesions, they can be grouped as high-flow (arteriovenous) and low-flow (capillary, cavernous, venous) lesions. Low-flow lesions are difficult to differentiate as they show similar MRI finding and often have overlapping pathologic appearances. Literature review showed that only seven cases of cavernomas have been reported in the hypothalamus.[2345]
Cerebral vascular malformations consist of arteriovenous malformation, arteriovenous venous fistula, cavernous malformation, and capillary telangiectasia. Depending on the predominant type of anomalous vascular channel at histopathology, vascular malformations are classified as capillary, cavernous, venous, and arteriovenous malformation.[6] Cavernomas are occult lesions on routine angiography since they are slow-flow venous malformations.[6] Cavernous malformations are collections of endothelium-lined sinusoids without intervening the cerebral parenchyma. While capillary telangiectasias are clusters of dilated capillaries interspersed in brain parenchyma, the developmental venous anomalies are veins arranged in a radial pattern, draining into a single larger vein. Arteriovenous malformations consist of pial arteries supplying a nidus which join together and drain into the venous system. In contrast, arteriovenous fistulas do not have an intervening nidus and are direct abnormal communication between cerebral arteries and veins.[6]
Hypothalamic cavernomas commonly present with headache and visual disturbance,[4] and in only one case, the patient presented with a history of memory loss.[3] On imaging, it was difficult to distinguish cavernoma from other low-flow vascular malformations due to their similar appearances. Conventional MRI sequences detect cavernomas based on the presence of hemorrhage. If the cavernoma has not bleed, then susceptibility-weighted imaging (SWI) sequences play a role, in their early detection. Computed tomography will detect hemorrhage or calcification due to the underlying cavernoma.[1]
MRI is the gold standard for cavernomas and findings depend on the stage and age of hemorrhage.[17] If cavernoma have not bleed, SWI sequence is preferred over other imaging techniques as it uses relaxation and magnetic susceptibility differences between oxygenated and deoxygenated blood of arterial and venous circulation to detect lesions.[8] However, the lesional size in SWI appears larger than their real size.[9]
Cavernous malformations can be observed or microsurgically managed. Radiosurgery is the newer alternative with very less chance of re-bleed and lower morbidity.[10] The uniqueness of this case report is in the susceptibility-weighted sequence, which led to the radiological diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Diagnosis and treatment of vascular malformations of the brain. Curr Treat Options Neurol. 2014;16:279.
- [Google Scholar]
- Cavernous malformations of the optic pathway and hypothalamus: Analysis of 65 cases in the literature. Neurosurg Focus. 2010;29:E17.
- [Google Scholar]
- Hypothalamic cavernous angioma associated with memory and behavior disturbance attacks: Role of imaging in diagnosis. Iran J Radiol. 2012;9:42-4.
- [Google Scholar]
- Exophytic hypothalamic cavernous malformation mimicking an extra-axial suprasellar mass. Emerg Radiol. 2011;18:363-7.
- [Google Scholar]
- Hemangioma from head to toe: MR imaging with pathologic correlation. Radiographics. 2004;24:367-85.
- [Google Scholar]
- Lesions of the hypothalamus: MR imaging diagnostic features. Radiographics. 2007;27:1087-108.
- [Google Scholar]
- Emerging clinical imaging techniques for cerebral cavernous malformations: A systematic review. Neurosurg Focus. 2010;29:E6.
- [Google Scholar]
- Susceptibility-weighted MR imaging: A review of clinical applications in children. AJNR Am J Neuroradiol. 2008;29:9-17.
- [Google Scholar]
- Stereotactic radiosurgery of intracranial cavernous malformations. Neurosurg Clin N Am. 2013;24:575-89.
- [Google Scholar]






