Translate this page into:
Automated intracranial hemorrhage detection in traumatic brain injury using 3D CNN
*Corresponding author: Deepak Agrawal, Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, India. drdeepak@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Agrawal D, Poonamallee L, Joshi S, Bahel V. Automated intracranial hemorrhage detection in traumatic brain injury using 3D CNN. J Neurosci Rural Pract 2023;14:615-21.
Abstract
Objectives:
Intracranial hemorrhage (ICH) is a prevalent and potentially fatal consequence of traumatic brain injury (TBI). Timely identification of ICH is crucial to ensure timely intervention and to optimize better patient outcomes. However, the current methods for diagnosing ICH from head computed tomography (CT) scans require skilled personnel (Radiologists and/or Neurosurgeons) who may be unavailable in all centers, especially in rural areas. The aim of this study is to develop a neurotrauma screening tool for identifying ICH from head CT scans of TBI patients.
Materials and Methods:
We prospectively collected head CT scans from the Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi. Approximately 738 consecutive head CT scans from patients enrolled in the department were collected for this study spanning a duration of 9 months, that is, January 2020 to September 2020. The metadata collected along with the head CT scans consisted of demographic and clinical details and the radiologist’s report which was used as the gold standard. A deep learning-based 3D convolutional neural network (CNN) model was trained on the dataset. The pre-processing, hyperparameters, and augmentation were common for training the 3D CNN model whereas the training modules were set differently. The model was trained along with the save best model option and was monitored by validation metrics. The Institute Ethics Committee permission was taken before starting the study.
Results:
We developed a 3D CNN model for automatically detecting the ICH from head CT scans. The screening tool was tested in 20 cases and trained on 200 head CT scans, with 99 normal head CT and 101 CT scans with some type of ICH. The final model performed with 90% sensitivity, 70% specificity, and 80% accuracy.
Conclusion:
Our study reveals that the automated screening tool exhibits a commendable level of accuracy and sensitivity in detecting ICH from the head CT scans. The results indicate that the 3D CNN approach has a potential for further exploring the TBI-related pathologies.
Keywords
Traumatic brain injury
3D convolutional neural network
Head computed tomography
Screening tool
Intracranial hemorrhage
INTRODUCTION
Annually, an estimated 64-74 million individuals across the globe are afflicted by traumatic brain injuries (TBI). India accounts for one-fourth of global deaths caused due to TBI.[1] Intracranial hemorrhage (ICH) is one of the severe consequences of TBI.[2] Traumatic injury on the head causes rupture of blood vessels inside the cranial vault, leading to abnormal accumulation of blood in the brain parenchyma or surrounding areas. ICH is categorized based on the location; however, it is imperative to diagnose ICH promptly as 50% of the associated mortalities occur within 24 hours of the injury. Early diagnosis and timely intervention are likely to enhance outcomes.[3-5]
Non-contrast head computed tomography (CT) scans are preferred for imaging due to their rapid acquisition and high sensitivity for identifying hemorrhages. Microhemorrhages are difficult to detect because they are present as small pockets of blood along the border of normal intracranial structures, they can confuse the neuroradiologist if the duration of the hemorrhage is spanning days or weeks. All the head CTs have to be visually examined by adjusting planes, level settings, and window widths. This task of manual interpretation heavily relies on the skill of the operator, and it is time-consuming. This can lead to imprecise or delayed diagnosis, which can be detrimental to the patient’s prognosis.[6,7]
There is an increasing interest in “automated” diagnostics in the medical field using advanced techniques such as deep learning. These techniques have proven efficacy in various medical imaging tasks, including neurology, pulmonary and thoracic diseases, ophthalmology, and more. With its growing success, artificial intelligence (AI) is being utilized in many aspects of healthcare, such as hospital management, virtual assistants, diagnostic image processing, drug discovery, and more.[8-10]
The focus of this article is on the implementation of automated techniques that can screen for abnormalities in medical imaging. It places specific emphasis on the application of convolutional neural network (CNN) algorithms to analyze head CT scans of TBI patients to detect instances of ICH. These methods have the potential of enhancing the efficiency and precision of ICH detection, which can lead to improved patient outcomes. To determine the advancements in neuromedicine, an exploration was conducted to identify research addressing the challenges faced by neurologists in remote regions and the necessity for automation in such contexts. A range of studies focusing on the development and validation of automated screening and quantification of TBI-related pathologies such as ICH, midline shift, and fracture using head CTs were identified.[11-14] We have extensively reviewed research that centers on automated ICH detection in cases of TBI.[15] It is noteworthy that most of these studies utilized training datasets with fewer than 200 head CT scans, which raises concerns about the robustness of the algorithms.[16] These advanced techniques for the detection of ICH can significantly improve the speed and precision of diagnosis and prognosis for TBI patients.[17]
Aims and objectives
The study aims to build a neurotrauma screening tool employing 3D CNN algorithms specifically for the detection of ICH in TBI patients.
MATERIALS AND METHODS
Data acquisition
The head CT scans were collected prospectively from the Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi. Ethics permission was taken from the Institute’s Ethics and Scientific Committee for the study. Consecutive head CT scans of patients visiting the neurosurgery department were used for the study over 9 months starting from January 2021 to September 2021. All CT scans were acquired from two machines, Siemens (Germany), and GE Healthcare (Illinois, United States of America). In the hospital, the head CT scans are archived in the standard Digital Imaging and Communications in Medicine format. Any attempt to retrieve and display CT images goes through Oviyam Picture Archives and Communication System (PACS) hardware.[17] The head CT scans were retrieved from the Oviyam PACS, and their consecutive clinical data were downloaded from the clinical patient record system (CPRS). The metadata collected along with the head CT scans consisted of demographic and clinical details (Glasgow coma score) as well as the radiologist’s report of the CT scans which were used as the gold standard.
Data were retrieved from an on-site PACS server and anonymized to comply with Health Insurance Portability and Accountability Act guidelines.
Clinical patient record system
After the data were acquired, it was analyzed to determine different aspects of the head CT scans collected. One such aspect was the slice thickness; it was ranging from 0.625 mm to 5 mm. Those with 1 mm slice thickness and 30 s kernel were included in the study. The head CT scans with artifacts are excluded from the study. True ICH cases are distinguished from normal cases through a knowledge-driven classification framework. The dataset encompasses distinct classes for training, validation, and testing. The training set consists of a total of 200 head CT scans, 99 normal cases and 101 cases with ICH. The distribution of the hemorrhage cases according to severity is as follows – mild – 20, moderate – 45, and severe – 35. The testing class consists of ten normal and ten hemorrhage cases. The total dataset, hence, is of 220 head CT scans.
Overall, this architecture is designed to efficiently extract and process important features from 3D images and produce accurate classification outputs.
Technique employed
The technique used here is 3D CNN. The convolutional neural layers are made up of fixed-sized convolutional filters that extract features from input images. These features are pooled and spatially compressed by a pooling layer, (max pooling/average pooling techniques). Thereafter, the features go through the fully connected layers, culminating in the output of the network.[6]
We have worked on developing the best-fit model based on the quantity and quality of the data. The model was trained along with the save best model option and was monitored by validation metrics. The pre-processing, hyperparameters, and augmentation were common for training the 3D CNN models whereas the training modules were set differently.
Implementation details
Labels
The binary label indicating the presence or absence of ICH for each head CT scan was assigned based on the “gold standard” radiologists’ reports.
Preprocessing
The dataset contains a variable number of 512 × 512 (pixel) images mainly due to head size differences and varying slice thicknesses. For uniformity, the whole dataset’s volume was resized to 128 × 128 × 64. Min-max normalization was performed on all the volumes.
Windowing with window level was set at 50 and window width was set at 150. The contrast was adjusted with a contrast factor of 2. Gaussian smoothing with sigma = 1. Augmentation was performed for training images by rotation to −20, −10, −5, 5, 10, and 20 angles.
Hyperparameters
The Batch size: 2. Initial learning rate: 0.0001 with exponential decay. Decay rate: 0.96. decay steps: 100,000, with staircase. 200 is the epoch limit with an early stopping mechanism. The loss function used for this model is binary cross-entropy, the learning rate was kept at 0.0001, and the Adam optimizer was used.
The training of the model is done by employing the binary cross-entropy loss function. The optimizer used is Adam due to its ability to adjust the model’s parameters during the training phase. The learning rate is set at 0.0001, governing the adjustments applied to the parameters during the training phase.
In addition, min-max normalization was applied to the volumes, which scales the voxel intensities to a range of (0,1). This normalization helps to ensure that the input data are in a consistent range and can increase the performance of the model during both the training and testing.
Architecture of model
[Figure 1] describes the model’s architecture; the structure is four blocks followed by a global average pooling layer, 2 fully connected dense layers, and a dropout layer.
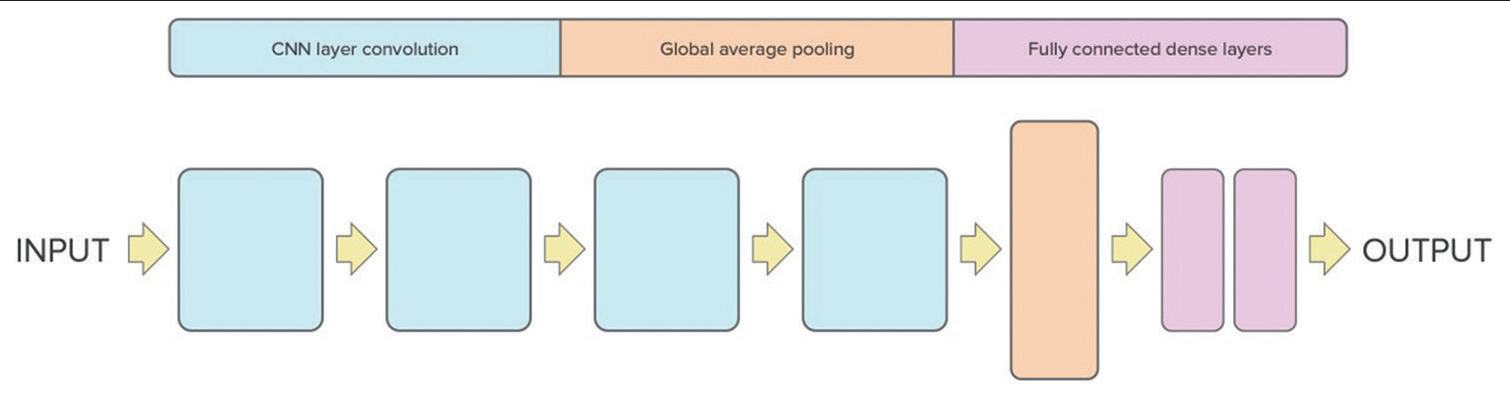
- Proposed 3D convolutional neural network architecture for intracranial hemorrhage screening.
The input of this CNN architecture is the head CT scans with dimensions (128, 128, and 64) and one channel. The architecture is comprised four blocks of 3D convolutional layers, each containing a convolutional layer followed by a rectified linear unit (ReLU) activation function, a 3D max-pooling layer along with batch normalization layer.
The first block reduces the dimensions of the input to 63, 63, 31, and 64, the second block further reduces the dimensions to 30, 30, 14, and 64, the third block reduces the dimensions to 14, 14, 6, and 128, and the fourth block reduces the dimensions to 6, 6, 2, and 256.
After the final block, a global average pooling layer is applied. This vector is then passed through a fully connected layer, followed by the integration of a dropout layer to counteract overfitting. Finally, the output layer has a single neuron that produces a binary classification output (1 or 0) indicating the presence or absence of hemorrhage in the head CT scan. The performance of the algorithm was evaluated by accuracy and loss metrics.
Training
The 3D CNN model was trained using three different training modules, each with a specific configuration.
In Training 1, the model was trained for 200 epochs which stopped at 60 epochs with a 60: 40 train: Val ratio, using a validation loss as the early stopping mechanism with a patience of 50.
In Training 2, the model was trained for 200 epochs which stopped at 50 epochs with a 70: 30 train: Val ratio, using a validation loss as the early stopping mechanism with a patience of 50.
In Training 3, the model was trained for 200 epochs which stopped at 48 epochs with a 70:30 train: Val ratio, using a validation accuracy of 0.8305 as the early stopping mechanism with a patience of 20 (10% of epochs).
RESULTS
Model accuracy
Our study demonstrates that 3D CNN, trained on clinical imaging datasets, exhibits the capability to accurately detect crucial radiological conditions such as ICH. The screening tool was tested on 20 cases and trained on 200 Head CT scans, with 99 normal head CT and 101 CT scans with some type of ICH. The evaluation of the training modules is shown in [Table 1].
| Accuracy | Specificity | Sensitivity | |
|---|---|---|---|
| Model 1 | 60% | 70% | 50% |
| Model 2 | 80% | 70% | 90% |
| Model 3 | 70% | 70% | 70% |
The Training 1 model was able to detect seven hemorrhage cases out of ten true hemorrhage cases and five normal cases out of ten true normal cases, achieving an accuracy of 60%, sensitivity of 50%, and specificity of 70%. The loss and accuracy were recorded as 0.4782 and 0.7333, respectively, while the validation loss and accuracy were recorded as 0.4828 and 0.7468, respectively.
The Training 2 model was able to detect seven ICH cases out of ten true ICH cases and nine normal cases out of ten normal cases. The loss and accuracy were recorded as 0.5654 and 0.6859, respectively, while the validation loss and accuracy were recorded as 0.4844 and 0.7627, respectively [Figure 3].
Whereas the Training 3 model was able to detect seven ICH cases out of ten ICH cases and seven normal cases out of ten normal cases, achieving an accuracy of 70%, sensitivity of 70%, and specificity of 70%. The loss and accuracy were recorded as 0.5548 and 0.6928, respectively, while the validation loss and accuracy were recorded as 0.4720 and 0.8305, respectively.
[Figure 2] illustrates the confusion matrix for the best-performing training plot. The Training 2 model had the best performance with a sensitivity of 90%, specificity of 70%, and 80% accuracy. The overall performance of our 3D CNN model indicates its potential to enhance the speed and precision of ICH detection.
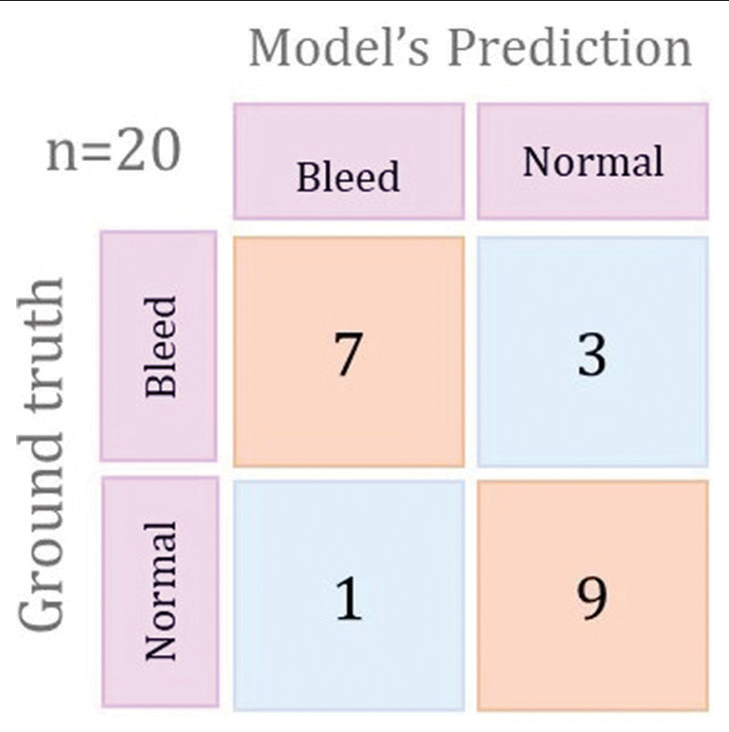
- Confusion matrix for the best performing training plot.
DISCUSSION
Our investigation delved into the creation of a 3D convolutional neural network-based approach to screen for ICH from head CT scans. The study showed that the automated screening tool is highly sensitive and fairly accurate in detecting ICH without a priori knowledge of their location or control of factors such as patient age and scanner type. [Figure 3] illustrates the 3D convolutional network-based approach which emulates the radiologist’s approach for screening of the ICH from head CT scans. The model’s prediction results were benchmarked against the “gold standard” radiologist’s reports.[6]
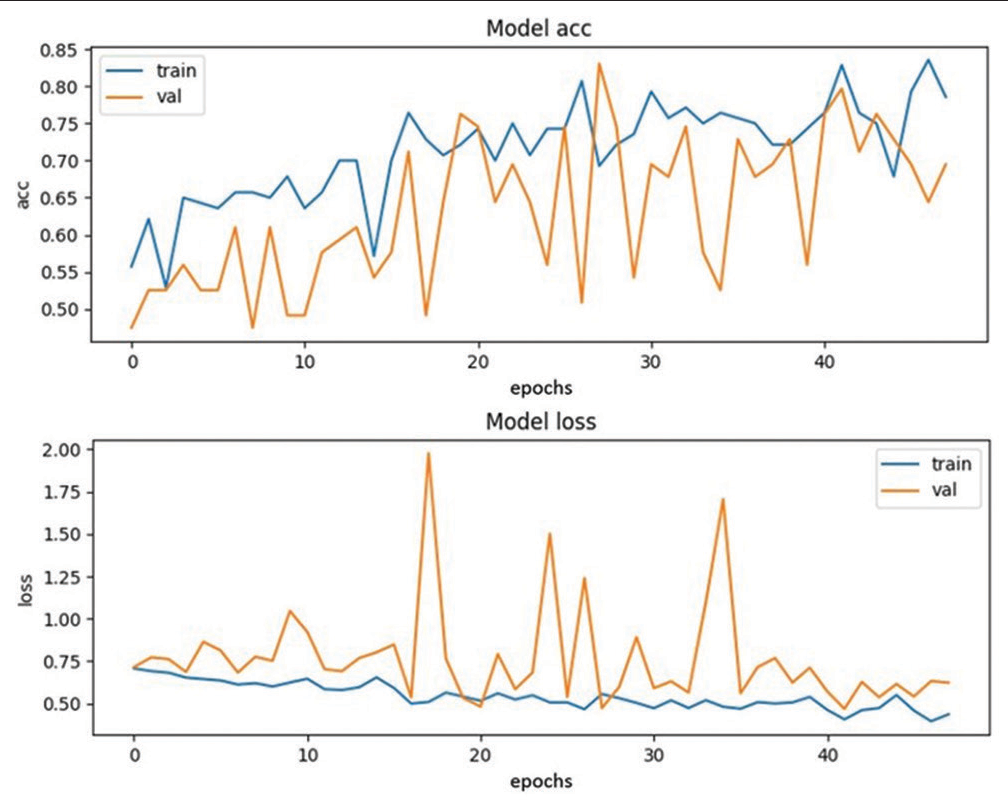
- Training Plot 2.
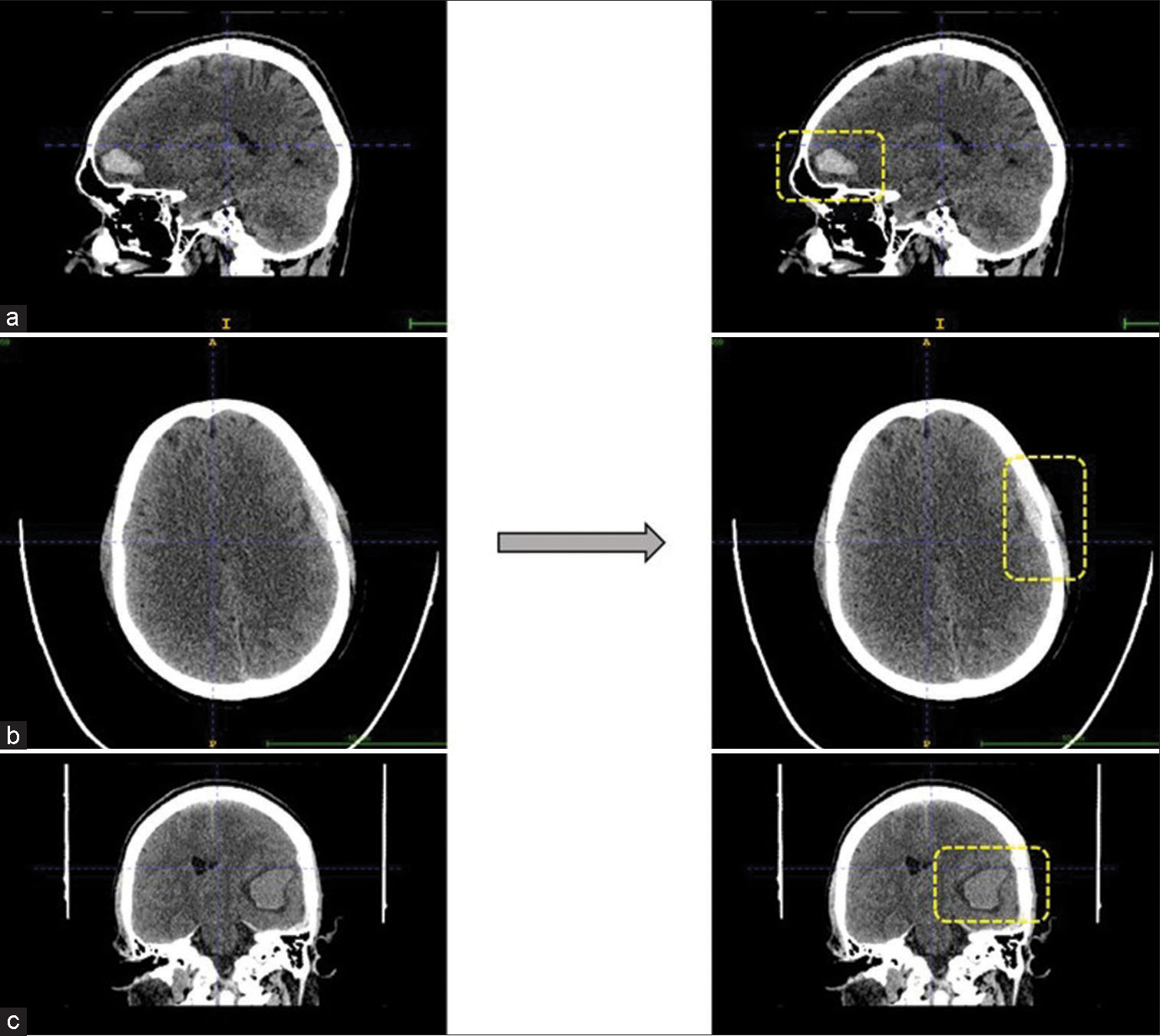
- Predicted intracranial hemorrhage bounded by the boxes. (a and c) are intraparenchymal hemorrhages seen in sagittal and coronal view, respectively. Whereas (b) is example for microsubdural hemorrhage seen in axial view.
There is a surge in interest surrounding CNN methods with several noteworthy research works using this technique for neuroradiology purposes. Apart from the screening of ICH, work is also done on automated classification and quantification of the ICH in TBI cases. One of the review articles mentioned the overall advancements made in neurology.[6] Yamada et al. focused on retrieving scans with fractures whereas Zaki et al. used computer vision for the purpose of detecting fractures. These techniques have also been utilized for automated midline shift detection.
A study by Chilamkurthy et al. undertook an extensive study by retrospectively collecting a huge dataset for automated detection of critical findings from CT scans.[18] This dataset comprised head CT scans and radiology reports that were collected over the span of 6 years from 20 centers span India. However, they choose to exclude the population below 7 years of age. The dataset collected for the current study is from one center over a shorter time span, providing data consistency, reduced confounding factors, time efficiency, and quality control. The exclusion of patients younger than 7 years limited the training based on a specific age range, whereas the dataset in this study comprised patients of various ages and genders. The study algorithm is thus applicable to all age groups and mixed genders.
This research work carries significant implications for the integration of AI techniques in clinical practices. However, there are several limitations to this study, including the use of radiological reports as a reference for extracting labels for each head CT, whose accuracy is unknown and could affect the model’s predictive accuracy. The high sensitivity level is an essential attribute of an automated approach in emergency scenarios.[19] Most of the studies have focused on segmentation and classification of ICH from the head CT scans which increase the time span required for the data pre-processing and algorithm development, whereas our algorithm just as its purpose was quickly developed and stat executed on the available sample set.
Few notable researchers have assessed the performance of deep learning to detect ICH, but the dataset they considered was smaller than our study. These studies can favorably be compared with our study due to the dataset parameter. Prevedello et al. evaluated a deep learning model’s performance on 76 CT images, aiming to identify ICH, hydrocephalus, mass effect, and acute infarct. Their findings revealed area under the curve (AUCs) of 0.91 for hemorrhage, mass effect, and hydrocephalus.[20] Another work by Grewal et al. introduced a deep learning approach focusing on the automated detection of ICH and used 77 head CT scans. Three radiologists validated the outputs and the results of the models indicated a sensitivity of 0.8864 and a positive predictive value of 0.8124.[19] In another study, Desai et al. proposed a deep learning model that utilized a pre-trained Google Net to identify basal ganglia hematoma presence in a dataset containing 170 CT images, achieving an AUC of 1.00.[12 ,21]
Some researchers have worked on the classification and quantification of the ICH using AI techniques and further discussion is about their works.[8,21,22] Hssayeni et al. introduced a fully automated U-Net model for the segmentation of ICH lesions from 82 CT scans and achieved a sensitivity of 97.28, specificity of 50.4, and dice coefficient of 0.31.[23] Irene et al. devised a dynamic graph convolutional neural network model tailored for ICH segmentation using 27 CT scans and achieved a sensitivity of 97.8% specificity: 95.6.[11,24] Another work on segmentation was done by Arab et al., using deep learning based on 64 CT scans and got a precision of 0.85 and a dice coefficient of 0.84.[25] Based on our current findings, we aim to direct our work toward automated segmentation using 3D CNN in the future.
The following studies have worked on large datasets and recorded their results.[26] Hence, the comparison is based on other parameters. In the study conducted by Titano et al., a 3D CNN model was developed based on ResNet-50 to categorize critical and non-critical CT findings. The number of datasets was 372236 Head CT scans and recorded 0.79 sensitivity and 0.48 specificity and 0.88 of AUC (without comparison to the gold standard). Despite the smaller dataset, our model’s performance is superior.[24] The effectiveness of finely tuned, pre-trained AlexNet-support vector machine (SVM) was demonstrated by Dawud et al. Their dataset comprised 12,635 head CTs and recorded an accuracy of 93.48, sensitivity of 95, and specificity of 90, they also classified hemorrhages in four classes.[27] Similarly, Kuo et al. proposed a patch-based and fully convolutional network capable of accurately segmenting and categorizing ICH with high accuracy.[28]
A majority of prior research has concentrated on the segmentation and classification of ICH in head CT scans. This often prolongs the time needed for data collection, preprocessing, and algorithm formulation. In contrast, the algorithm in our study was swiftly developed and implemented using the accessible sample dataset.
In conclusion, the integration of the 3D CNN model into radiology worklists has the capacity to significantly expedite ICH, saving valuable time and potentially uncovering subtle ICH cases that might have been overlooked by human radiologists. This underlines the positive influence of AI in optimizing radiology workflows. The timing of diagnosis is inherently tied to the efficiency of completing a head CT scan and subsequently interpreting it by a medical professional.[5,18] Notably, it is essential to emphasize that a high level of sensitivity is a pivotal attribute for automated systems intended for emergency diagnostic applications.
Future directions and limitations
This work deals only with the ICH detection as whole and not individual categories of the ICH. The model is not a diagnosis tool and does not classify the ICH based on its location. Expanding the dataset and training the 3D CNN model further will improve the outcomes of this study. Training the model on various classes of hemorrhages can increase the scope to detect as well as classify the ICH cases. Inclusion of any slice thickness and any kernel as well as more data will make the model more robust and boost up the accuracy.
CONCLUSION
In this study, we found that the automated screening tool has good sensitivity for ICH detection on head CT in patients with TBI. The outcomes of employing the 3D CNN technique point toward the potential of automated ICH detection for integration in the clinical workflow. It can also play a role for educational and research purposes and navigate through extensive CT datasets. It is crucial to acknowledge that further investigations are essential to overcome the limitations and ensure the model’s effectiveness across diverse patient groups and imaging equipment setups.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Financial support and sponsorship
Nil.
References
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-858.
- [CrossRef] [PubMed] [Google Scholar]
- Position statement: Definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91:1637-40.
- [CrossRef] [PubMed] [Google Scholar]
- Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130:1080-97.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial bleeding in patients with traumatic brain injury: A prognostic study. BMC Emerg Med. 2009;9:15.
- [CrossRef] [PubMed] [Google Scholar]
- Advanced machine learning in action: Identification of intracranial hemorrhage on computed tomography scans of the head with clinical workflow integration. NPJ Digit Med. 2018;1:9.
- [CrossRef] [PubMed] [Google Scholar]
- Automated detection and screening of Traumatic Brain Injury (TBI) using computed tomography images: A comprehensive review and future perspectives. Int J Environ Res Public Health. 2021;18:6499.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-10.
- [CrossRef] [PubMed] [Google Scholar]
- Automatic quantification of computed tomography features in acute traumatic brain injury. J Neurotrauma. 2019;36:1794-803.
- [CrossRef] [PubMed] [Google Scholar]
- Weakly supervised learning significantly reduces the number of labels required for intracranial hemorrhage detection on head CT Vol arXiv. 2022.
- [Google Scholar]
- Secure and robust machine learning for healthcare: A survey. IEEE Rev Biomed Eng. 2021;14:156-80.
- [CrossRef] [PubMed] [Google Scholar]
- Segmentation and approximation of blood volume in intracranial hemorrhage patients based on computed tomography scan images using deep learning method In: International workshop on big data and information security (IWBIS). United states: IEEE; 2020. p. :65-72.
- [CrossRef] [Google Scholar]
- Application of deep learning in neuroradiology: Automated detection of basal ganglia hemorrhage using 2D-convolutional neural networks Vol arXiv. 2017.
- [Google Scholar]
- Synergic deep learning model-based automated detection and classification of brain intracranial hemorrhage images in wearable networks. Pers Ubiquitous Comput. 2022;26:1-10.
- [CrossRef] [Google Scholar]
- Segmenting hemorrhagic and ischemic infarct simultaneously from follow-up non-contrast CT images in patients with acute ischemic stroke. IEEE Access. 2019;7:39842-51.
- [CrossRef] [Google Scholar]
- Automated detection of intracranial hemorrhage from head CT scans applying deep learning techniques in traumatic brain injuries: A comparative review. Indian J Neurotrauma. 2023;20:81-8.
- [CrossRef] [Google Scholar]
- Detecting intracranial hemorrhage with deep learning. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:583-7.
- [CrossRef] [PubMed] [Google Scholar]
- Automatic detection and classification of brain hemorrhages. WSEAS Trans Comput. 2013;12:395-405.
- [Google Scholar]
- Deep learning algorithms for detection of critical findings in head CT scans: A retrospective study. Lancet. 2018;392:2388-96.
- [CrossRef] [PubMed] [Google Scholar]
- RADNET: Radiologist level accuracy using deep learning for HEMORRHAGE detection in CT scans. United States: IEEE; 2017
- [CrossRef] [Google Scholar]
- Automated critical test findings identification and online notification system using artificial intelligence in imaging. Radiology. 2017;285:923-31.
- [CrossRef] [PubMed] [Google Scholar]
- Automated subdural hematoma segmentation for Traumatic Brain Injured (TBI) patients. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:3069-72.
- [CrossRef] [PubMed] [Google Scholar]
- Automated hematoma segmentation and outcome prediction for patients with traumatic brain injury. Artif Intell Med. 2020;107:101910.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial hemorrhage segmentation using a deep convolutional model. Data. 2020;5:14.
- [CrossRef] [Google Scholar]
- Automated deep-neural-network surveillance of cranial images for acute neurologic events. Nat Med. 2018;24:1337-41.
- [CrossRef] [PubMed] [Google Scholar]
- A fast and fully-automated deep-learning approach for accurate hemorrhage segmentation and volume quantification in non-contrast whole-head CT. Sci Rep. 2020;10:19389.
- [CrossRef] [PubMed] [Google Scholar]
- Computer aided detection of small acute intracranial hemorrhage on computer tomography of brain. Comput Med Imaging Graph. 2007;31:285-98.
- [CrossRef] [PubMed] [Google Scholar]
- Application of deep learning in neuroradiology: Brain haemorrhage classification using transfer learning. Comput Intell Neurosci. 2019;2019:4629859.
- [CrossRef] [PubMed] [Google Scholar]
- Expert-level detection of acute intracranial hemorrhage on head computed tomography using deep learning. Proc Natl Acad Sci U S A. 2019;116:22737-45.
- [CrossRef] [PubMed] [Google Scholar]







