Translate this page into:
Association between Foramen Size and Febrile Seizure Status in the Pediatric Population: A Two-Center Retrospective Analysis
Peyton Presto, MS, School of Medicine Texas Tech University Health Sciences Center 5202 Auburn Street, Apt 1927, Lubbock, TX 79416 United States Peyton.presto@ttuhsc.edu
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objective Febrile seizures have been shown to occur in 2 to 5% of children between the ages of 6 months and 5 years, making them the most common seizures of childhood. Multiple risk factors for febrile seizures have been identified; however, no investigation has been conducted to explore foramen size and associated venous drainage as a potential risk factor for experiencing febrile seizures. Of particular interest are the parietal foramen and the condylar canal, which conduct the parietal emissary vein and the occipital emissary vein, respectively. Emissary veins lack valves, allowing them to play a crucial role in selective brain cooling via a bidirectional flow of blood from the head’s evaporating surface. Narrowed cranial apertures conducting these veins may lead to reduced cerebral venous outflow and delayed brain cooling, creating favorable conditions for a febrile event. This study seeks to explore the association between cranial aperture area and febrile seizure status.
Methods A retrospective cross-sectional medical record review study from January 2011 to December 2017 was conducted at a 500-bed academic hospital and a 977-bed private hospital in Lubbock, Texas, United States. A total of 101 complex febrile seizure patients were compared with a similarly aged group of 75 trauma patients representing the normal population. Parietal foramen area and condylar canal area were electronically measured and defined as having “normal” or “below normal” area.
Statistical Analysis Independent t-tests were used to compare foramen and canal areas by febrile seizure status. Logistic regression analyses were conducted to determine the association of small cranial aperture area with febrile seizure status.
Results Below normal parietal foramen area had a strong association with febrile seizures in our patient population. Male sex, white race, and complete vaccination status were also found to have significant associations with febrile seizure status.
Conclusion Our findings indicated that narrowed parietal foramen may be considered as a risk factor for febrile seizure development.
Keywords
cerebral blood flow
febrile seizures
neuroanatomy
neuroimaging
pediatrics
risk factors
Introduction
Febrile seizures are an acute neurological condition in children. With a 2 to 5% occurrence rate, they are the most common form of childhood seizure and account for a large portion of emergency department visits each year.1 2 3 4 The American Academy of Pediatrics (AAP) defines a febrile seizure as “a seizure accompanied by a fever (temperature >100.4°F or 38°C by any method), without central nervous system infection, that occurs in infants and children at the age of 6 to 60 months.”5 Febrile seizures may be classified as simple or complex based on duration, nature, and rate of occurrence.1 2 3 4 6 Whereas simple febrile seizures are generalized in nature and have a brief duration with no reoccurrence—complex febrile seizures are longer—may be accompanied by focal symptoms and can recur within 24 hours.1 2 3 4 6 7 Current risk factors for febrile seizures include age, male sex, vaccination status, nutritional deficiencies, family history, duration of initial seizure, developmental delay, and temperature.1 2 3 4 6 7 8 9 Since the pathogenesis of febrile seizures involves elevated body temperature,1 2 3 4 7 an investigation into the relationship between cooling mechanisms of the brain—emissary veins and their respective canals—and occurrence of febrile seizure is warranted.
In a hyperthermic state, blood in the emissary veins flows into the skull (opposite the direction of normal venous outflow), allowing cooler blood to reduce elevated brain temperatures.10 We predicted that narrowed areas of the associated canals—specifically the condylar canals and the parietal foramina—could prevent sufficient blood flow from reaching the brain, predisposing an individual to febrile seizures. Here, we investigated the relationship between the areas of these regions as measured by computed tomography (CT) imaging with the occurrence of complex febrile seizures in a pediatric cohort.
Methods
Patient Selection
This retrospective study had institutional review board approval with a waiver of the informed consent requirement. We isolated a group of 552 patients who were diagnosed as having a febrile seizure (simple or complex) at either University Medical Center (UMC) or Covenant Medical Center (CMC) in Lubbock, Texas, United States, between January 2011 and December 2017. Febrile seizure cases were identified by International Statistical Classification (ICD)-9 (780.31–780.32) and ICD-10 (R56.00–R56.01) codes. From these cases, patients between the ages of 6 and 60 months at diagnosis were selected for our initial cohort (in accordance with the AAP febrile seizure definition5). The patients’ MRN number was then replaced with generic numerical substitutes (1, 2, 3, etc.) for declassification. Subjects were excluded if they had prior neurosurgical intervention, preexisting neurologic or cranial structural anomalies, prior diagnosis of nonfebrile epileptic activity, coexisting diagnoses of febrile seizure and injury due to external causes, or were current recipients of antiepileptic or known epileptogenic medications.
Though our initial cohort included both simple and complex febrile seizure diagnoses, we found neuroimaging to be available for only the complex febrile seizure subgroup. We then narrowed our cohort to include just complex febrile seizure diagnoses. For subjects with more than one encounter, data were only collected for the most recent encounter meeting study eligibility criteria.
Measurements
The following demographic/categorical data were collected: age, sex, race/ethnicity, and ICD-9 and ICD-10 codes. The patient’s head circumference, head circumference percentile, and presence of any previously mentioned known risk factors for febrile seizures—male sex, vaccination status, nutritional deficiencies, family history, and developmental delay—were also documented.7 8 9 We then measured the parietal foramina and condylar canal dimensions from this group of patients through the use of electronic calipers and the freehand analysis tool on head CT imaging software. Measurements were repeated three times for each foramen or canal visualized on imaging software (left only, right only, or bilateral), and the average of these values was taken as a single, final value for each canal/foramen area.
Classification
Since we were interested in the possibility of a correlation between reduced canal/foramina area and increased febrile seizure occurrence, both canal and foramen areas were classified as “below normal range” or “within normal range” for each patient. There is much discrepancy in the literature regarding a normal range for these values due to widely accepted anatomical variations in both cranial apertures. Studies have found the condylar canals to have a mean 3.5 mm diameter,11 but little has been discussed with regard to mean area. The parietal foramen have been suggested to have an average diameter of 0.5 mm with greater than 1.5 mm being rare.12 Others described parietal foramina exceeding 5 mm in diameter as enlarged.13 The parietal foramen may also be influenced by sexual dimorphism, as females skulls have been found to have parietal foramina twice as large as their male counterparts (3 mm in females and 1.5 mm in males).14 15 A similar trend has not been exhibited in condylar canal measurements. We elected to use the mean areas and standard deviations as described by Wysocki et al, which found a mean area of 12.61 ± 8.97 mm2 for the condylar canals and 2.03 ± 1.24 mm2 for the parietal foramen.15 Values that fell within these ranges were classified as “normal,” whereas values that fell below the lower end of this range (3.64 and 0.79 mm2 for the condylar canals and parietal foramen, respectively) were classified as “below normal.”
Trauma Control Group
The same information and measurements as detailed in the “measurements” and “classification” sections were collected for a group of 75 similarly aged patients seen in the UMC or CMC emergency departments over the same time period. These patients were initially assessed for trauma but found to have no trauma-induced anatomical skull deformities on neuroimaging. Patients were excluded from this cohort if they had experienced any prior neurosurgical intervention, had preexisting neurologic or cranial structural anomalies, or been diagnosed with a febrile seizure. This group represented the normal population and was used as a control group for the purposes of this study.
Statistical Analysis
Independent t-tests were used to compare foramen and canal areas by febrile seizure status. Logistic regression analyses were used to estimate multivariate (adjusted) odds ratios with 95% confidence intervals for all categorical variables (sex, vaccination status, race, family history of seizure, and foramen/canal size categories) in the model.
Results
Of the 552 patients who fit our initial inclusion criteria, 451 were excluded due to lack of head CT imaging or inadequate image quality from patient movement. Of the 329 trauma patients who fit the control group inclusion criteria, 254 were excluded for similar reasons. The mean age of the 101 remaining febrile seizure patients (32 females and 69 males) was 21 months, and the mean age of the 75 remaining trauma patients (40 females and 35 males) was 38 months. Categorical characteristics and presence of known risk factors for febrile seizures are listed in Table 1.
|
Overall, % (number of patients) |
Trauma, % (number of patients) |
Febrile seizure, % (number of patients) |
p-Value |
|
|---|---|---|---|---|
|
Age in mo (range) |
24 (16–48) |
38 (23–60) |
21 (14–32) |
<0.0001 |
|
Sex |
0.004 |
|||
|
Female |
40.9 (72) |
22.7 (40) |
18.2 (32) |
|
|
Male |
59.1 (104) |
19.9 (35) |
39.2 (69) |
|
|
Race |
<0.0001 |
|||
|
White |
34.0 (55) |
21.6 (35) |
12.4 (20) |
|
|
Hispanic |
66.1 (107) |
20.4 (33) |
45.7 (74) |
|
|
Recurrent febrile seizure |
<0.0001 |
|||
|
No |
68.8 (121) |
42.6 (75) |
26.1 (46) |
|
|
Yes |
31.3 (55) |
0 (0) |
31.3 (55) |
|
|
Vaccination |
<0.0001 |
|||
|
No |
10.8 (19) |
8.5 (15) |
2.3 (4) |
|
|
Yes |
89.2 (157) |
34.1 (60) |
55.1 (97) |
|
|
Family history of seizures |
0.001 |
|||
|
No |
82.4 (145) |
39.8 (70) |
42.6 (75) |
|
|
Yes |
17.6 (31) |
2.8 (5) |
14.8 (26) |
|
|
Developmental delay |
0.103 |
|||
|
No |
84.7 (149) |
34.1 (60) |
50.6 (89) |
|
|
Yes |
15.3 (27) |
8.5 (15) |
6.8 (12) |
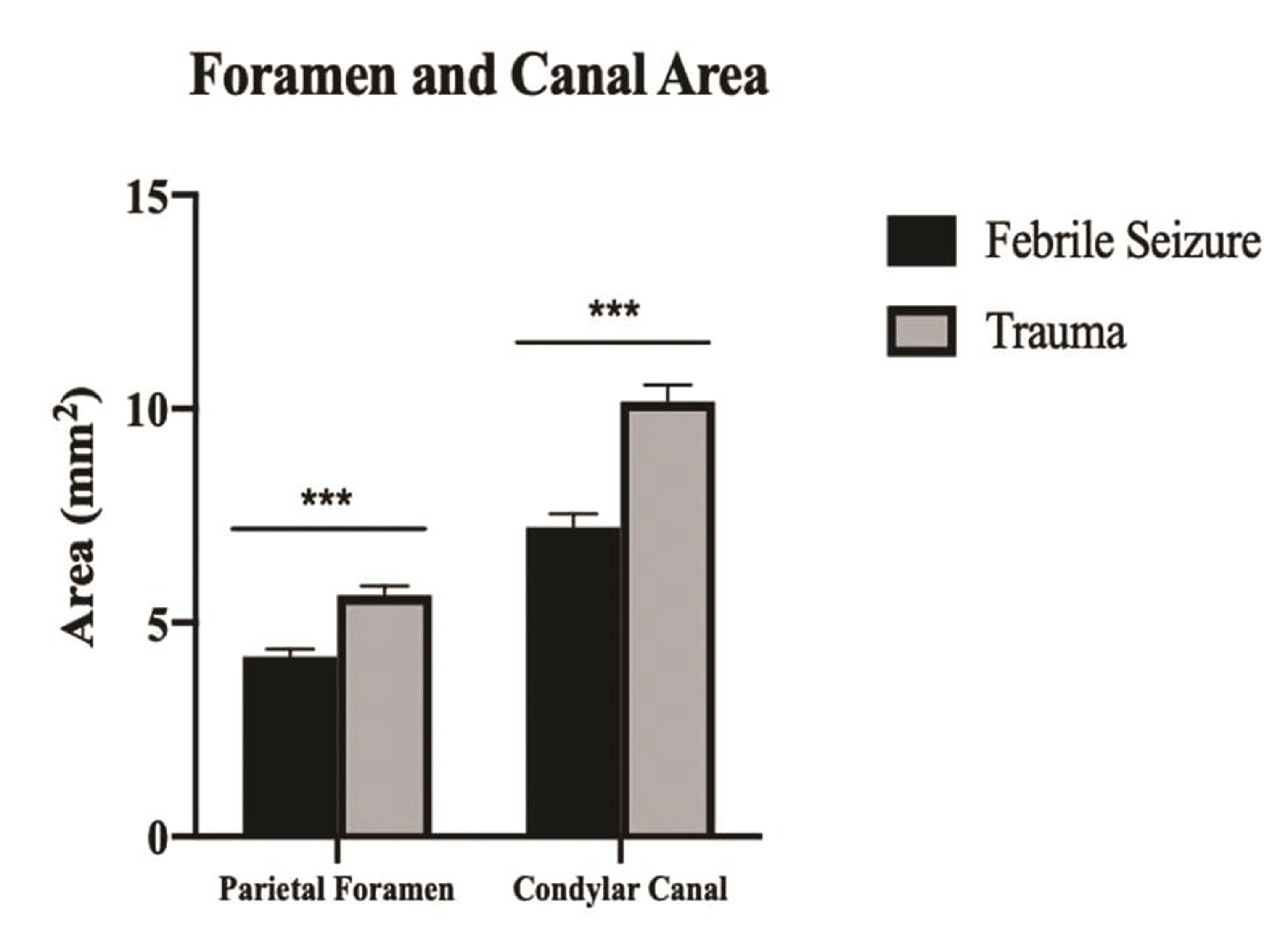
Classification of foramen and condylar canal size in our patient population is listed in Table 2. Of the 101 febrile seizure patients included in this study, 70 patients (69.3%) fit the criteria for below normal parietal foramen area, compared with 30 of the 75 trauma patients (40.0%; positive predictive value = 70%). Additionally, 9 of the 101 febrile seizure patients (8.9%) and no trauma patients were classified as having below normal condylar canal area. Average foramen and canal areas by febrile seizure status are shown in Fig. 1. Comparisons of condylar canals and of parietal foramina (illustrated by yellow arrows on images) are shown in Figs. 2 3, respectively.
|
Overall, % (number of patients) |
Trauma, % (number of patients) |
Febrile seizure, % (number of patients) |
p-Value |
|
|---|---|---|---|---|
|
Parietal foramen size |
<0.0001 |
|||
|
Below normal |
56.8 (100) |
17.1 (30) |
39.8 (70) |
|
|
Normal |
43.2 (76) |
25.6 (45) |
17.6 (31) |
|
|
Condylar canal size |
0.003 |
|||
|
Below normal |
86.4 (152) |
33.0 (58) |
53.4 (94) |
|
|
Normal |
13.6 (24) |
9.7 (17) |
4.0 (7) |
-
Fig. 1 Parietal foramen and condylar canal area by febrile seizure status. Significant differences were found between febrile seizure patients and trauma patients representing a normal population for both parietal foramen and condylar canal area; ***p < 0.001.
Fig. 1 Parietal foramen and condylar canal area by febrile seizure status. Significant differences were found between febrile seizure patients and trauma patients representing a normal population for both parietal foramen and condylar canal area; ***p < 0.001.
-
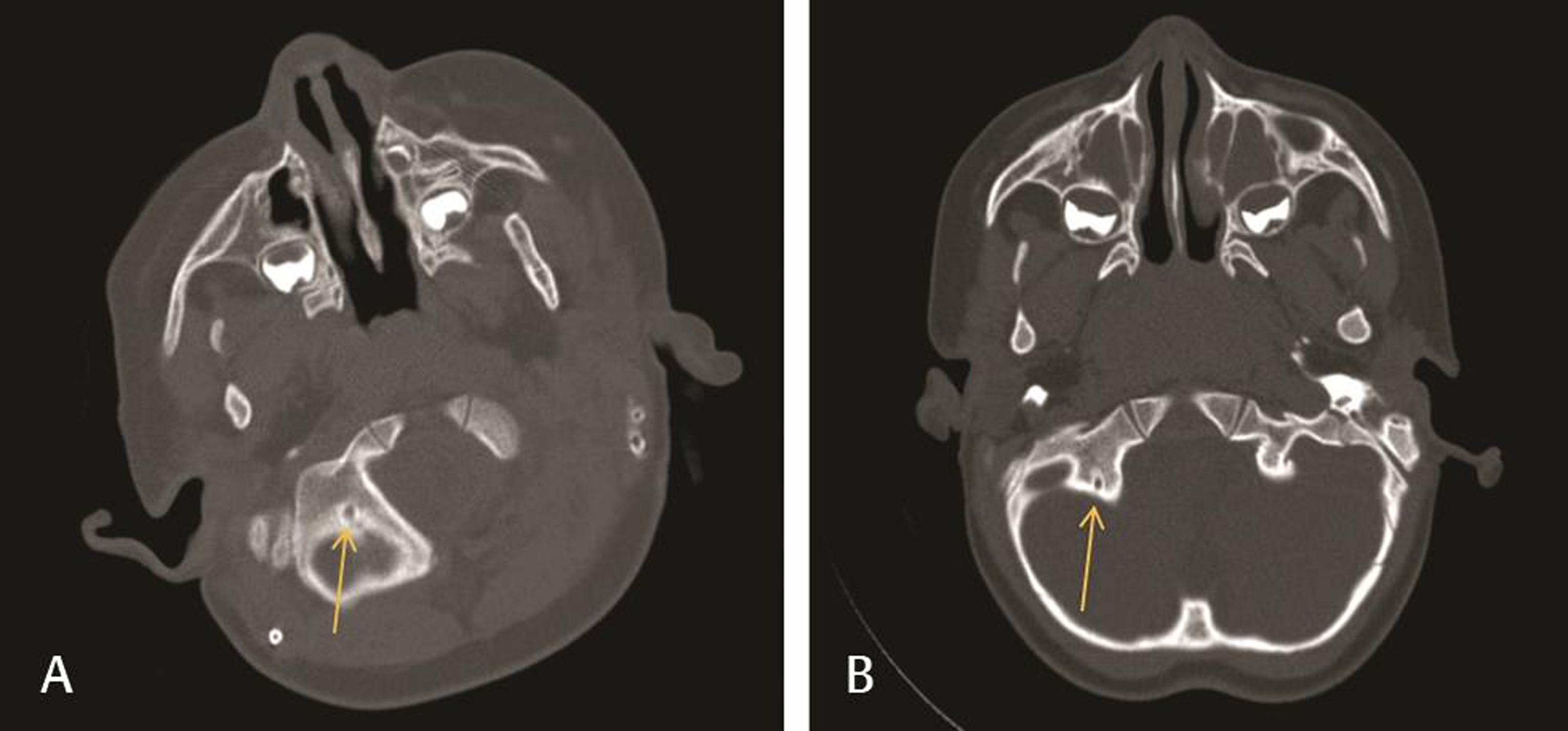
Fig. 2 Comparison of normal (A) and below normal (B) condylar canals on head CT imaging. (A) Head CT of a patient in the nonfebrile seizure trauma group used to illustrate a condylar canal that fit our criteria for normal size (12.57 mm2 shown here). (B) Head CT of a patient in the febrile seizure group used to illustrate a below normal condylar canal size (2.63 mm2 shown here). CT, computed tomography.
Fig. 2 Comparison of normal (A) and below normal (B) condylar canals on head CT imaging. (A) Head CT of a patient in the nonfebrile seizure trauma group used to illustrate a condylar canal that fit our criteria for normal size (12.57 mm2 shown here). (B) Head CT of a patient in the febrile seizure group used to illustrate a below normal condylar canal size (2.63 mm2 shown here). CT, computed tomography.
-
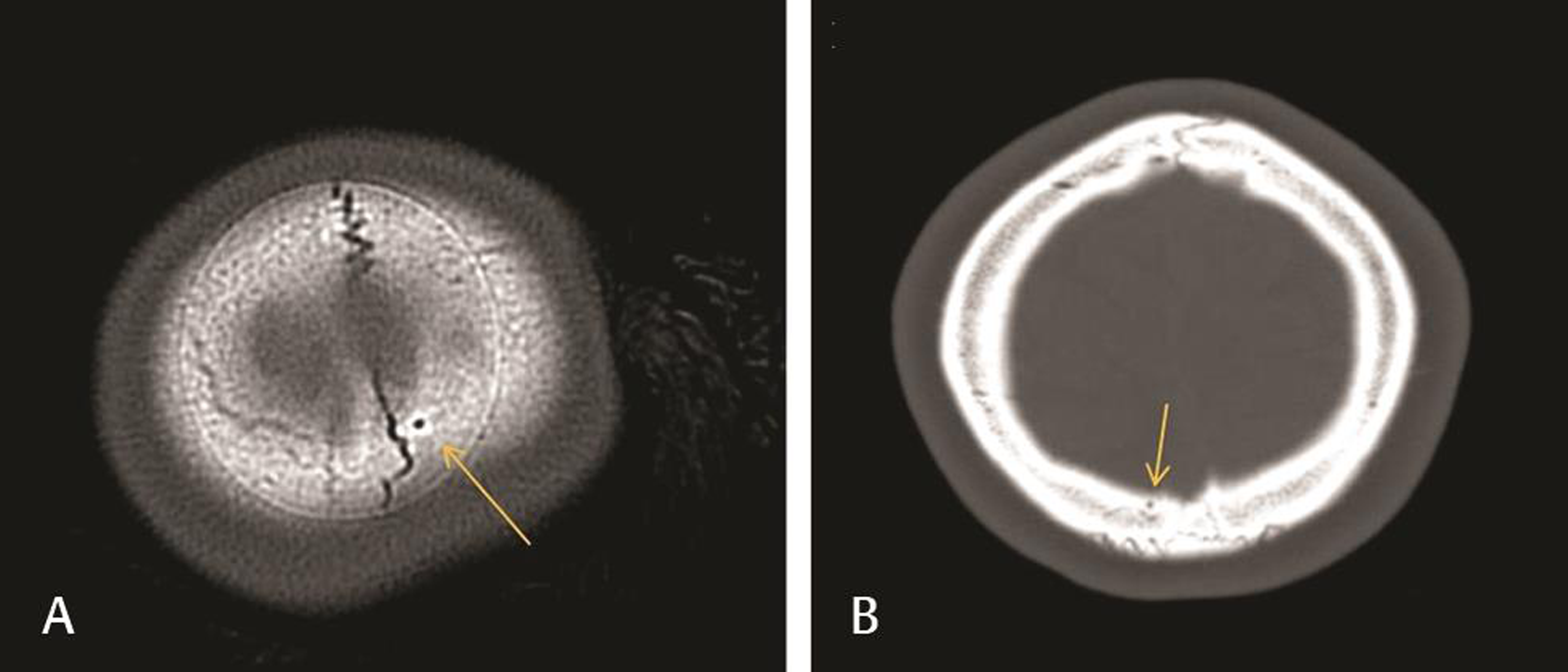
Fig. 3 Comparison of normal (A) and below normal (B) parietal foramina on head CT imaging. (A) Head CT of a patient in the nonfebrile seizure trauma group used to illustrate a parietal foramen that fit our criteria for normal size (2.80 mm2 shown here). (B) Head CT of a patient in the febrile seizure group used to illustrate a below normal parietal foramen size (0.74 mm2 shown here). CT, computed tomography.
Fig. 3 Comparison of normal (A) and below normal (B) parietal foramina on head CT imaging. (A) Head CT of a patient in the nonfebrile seizure trauma group used to illustrate a parietal foramen that fit our criteria for normal size (2.80 mm2 shown here). (B) Head CT of a patient in the febrile seizure group used to illustrate a below normal parietal foramen size (0.74 mm2 shown here). CT, computed tomography.
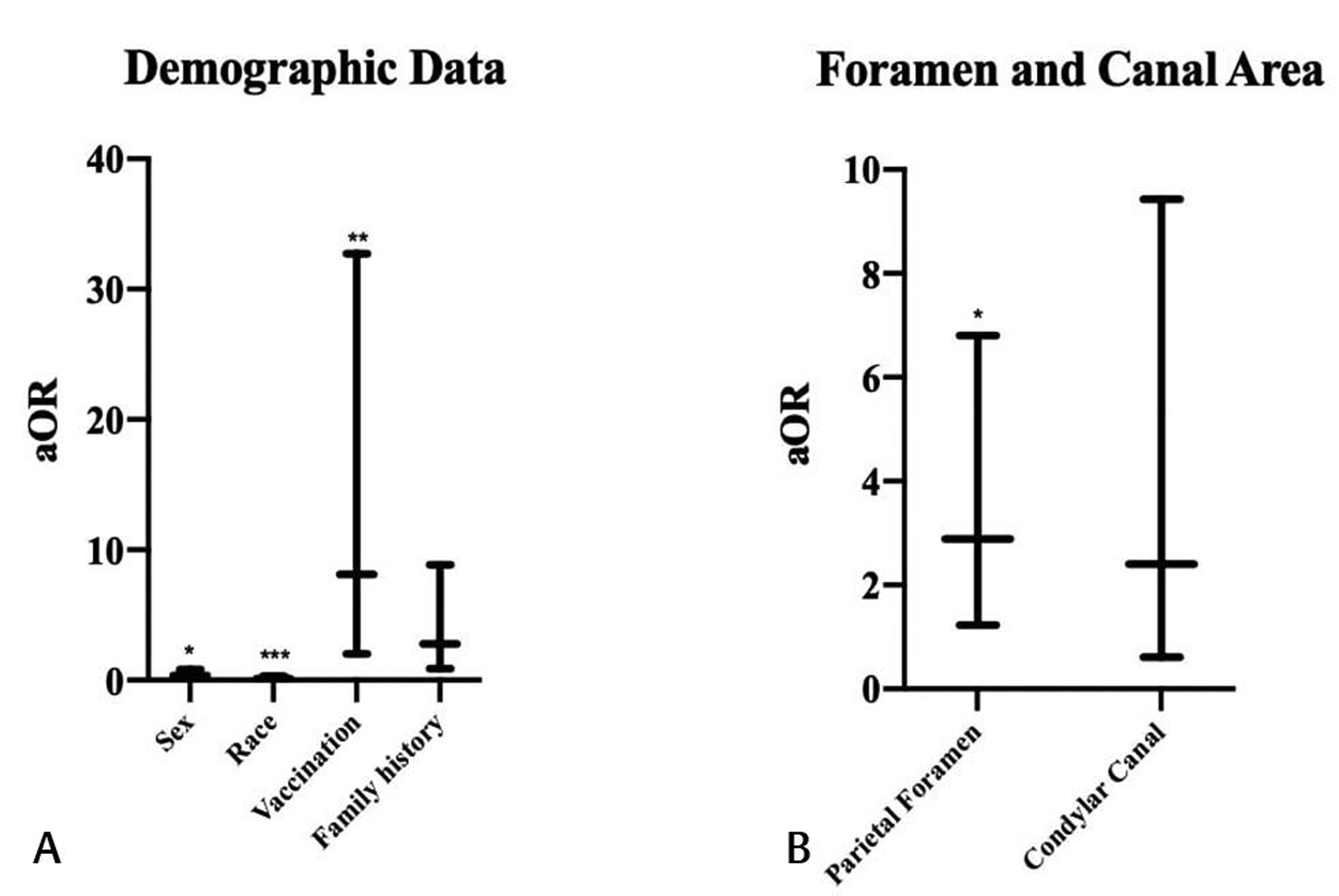
Adjusted odds ratios with 95% confidence intervals are shown in Fig. 4 and Table 3. Male sex (p = 0.016), white race (p < 0.001), complete vaccination status (p = 0.003), and presence of a below normal parietal foramen area (p = 0.015) were significantly associated with the development of febrile seizures. Having a family history of febrile seizures (p = 0.082) and below normal condylar canal area (p = 0.207) were not found to have significant associations. Head circumference, head circumference percentile, nutritional deficiencies, and developmental delay were excluded from analysis due to incomplete documentation in multiple patients’ medical records.
|
Adjusted odds ratio |
95% confidence interval |
p-Value |
|
|---|---|---|---|
|
Sex |
|||
|
Male (reference) |
|||
|
Female |
0.35 |
(0.15–0.82) |
0.016 |
|
Race |
|||
|
White (reference) |
|||
|
Hispanic |
0.13 |
(0.05–0.33) |
<0.001 |
|
Vaccination status |
|||
|
Incomplete (reference) |
|||
|
Complete |
8.12 |
(2.01–32.73) |
0.003 |
|
Family history of seizure |
|||
|
No (reference) |
|||
|
Yes |
2.79 |
(0.87–8.85) |
0.082 |
|
Parietal foramen area |
|||
|
Normal (reference) |
|||
|
Below normal |
2.89 |
(1.23–6.80) |
0.015 |
|
Condylar canal area |
|||
|
Normal (reference) |
|||
|
Below normal |
2.40 |
(0.61–9.43) |
0.207 |
-
Fig. 4 Adjusted odds ratios by febrile seizure status (A, B). We used the following criteria as reference data: male sex, white race, incomplete vaccinations, no family history of febrile seizure, normal parietal foramen area, and normal condylar canal area. Using this model, male sex, white race, complete vaccination status, and presence of a below normal parietal foramen area were significantly associated with the development of febrile seizures; *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 4 Adjusted odds ratios by febrile seizure status (A, B). We used the following criteria as reference data: male sex, white race, incomplete vaccinations, no family history of febrile seizure, normal parietal foramen area, and normal condylar canal area. Using this model, male sex, white race, complete vaccination status, and presence of a below normal parietal foramen area were significantly associated with the development of febrile seizures; *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Male sex, complete vaccination status, white race, and below normal parietal foramen area were significantly associated with the occurrence of febrile seizures in our patient population.
Although some studies have not shown a clear sex predilection for the occurrence of febrile seizures,2 6 this study demonstrated a significant association between male sex and febrile seizures. Other literature reports males to have a higher frequency of febrile seizures,3 16 17 18 19 which is more consistent with our findings. This may be attributed to sexual dimorphism in parietal ossification, as males have been shown to have smaller average parietal foramen sizes than females.14 15
The significant association between vaccination status and febrile seizure occurrence that is demonstrated in this study has been well documented in the literature.2 3 7 20 Although vaccine-induced febrile seizures do not lead to deleterious outcomes, they are the second most common medical event associated with the condition.3 20 None of the standard vaccinations are currently contraindicated in patients with febrile seizures.20 21 22
As for the significance of race and febrile seizure development, the literature reports a lower incidence of febrile seizures within the white race relative to Asian populations (3–5% in whites vs. 8–10% in Asians).2 3 6 However, a limitation of this study is the predominately white and Hispanic demographic within our sample, which limits generalizability to other racial/ethnic groups. It has been shown that Hispanic children have lower odds of being severely affected by febrile seizures than white children23; however, another study found increased incidence of febrile seizures in Hispanic versus non-Hispanic populations but no significance between race and febrile seizure duration.24
The mean age of patients who experienced febrile seizures within this study is 21 months, which is also in accordance with ages reported in the literature—reported febrile seizures occur between 3 months and 5 years of age,1 2 3 4 7 with a peak age between 18 and 24 months.1 2 3 6
Many studies have argued against the use of neuroimaging for first-time simple febrile seizures.1 2 3 7 25 The predominant argument opposing CT scanning is the escalating future cancer risk due to radiation exposure.3 7 25 The incidence of a clinically significant CT abnormality in complex febrile seizure patients has been reported to be as low as 1.6 to 5.0%.26 27 Teng et al found that in 71 patients experiencing a first complex febrile seizure—none required emergency neurosurgical or medical intervention—indicating that neuroimaging posed an unnecessary risk.7 28 However, current guidelines state that neuroimaging may be considered in febrile seizure patients presenting with neurologic abnormalities or in those with recurrent febrile seizures.2 3 7 If previously existing neuroimaging is available for such patients, a repeated examination of parietal foramen that reveals a small foramen area may be considered as a possible pathologic cause. Future replication of these results in cohorts with differing population characteristics is needed to further support the validity of foramen size as a risk factor for febrile seizure development.
Conclusion
Below normal parietal foramen area is significantly associated with febrile seizure development in our patient population. Our findings suggested that larger multicenter retrospective analyses may be necessary in febrile seizure patients with prior neuroimaging (related or unrelated) before considering any changes to febrile seizure management protocols.
Acknowledgments
The authors wish to acknowledge the contribution of the Texas Tech University Health Sciences Center Clinical Research Institute for their assistance with this research.
Note
This study was approved by the Institutional Review Board.
Conflict of Interest
None declared.
References
- Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121(6):1281-1286.
- [Google Scholar]
- Febrile Seizures.Encyclopedia of The Neurological Sciences (Second Edition) Academic Press; 2014. p. :281-282. In: eds.
- [Google Scholar]
- Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician. 2012;85(2):149-153.
- [Google Scholar]
- Risk factors for a first febrile seizure: a matched case-control study. Epilepsia. 1995;36(4):334-341.
- [Google Scholar]
- Hominid evolution of the arteriovenous system through the cranial base and its relevance for craniosynostosis. Childs Nerv Syst. 2007;23(12):1367-1377.
- [Google Scholar]
- Posterior condylar canals and posterior condylar emissary veins-a microsurgical and CT anatomical study. Neurosurg Rev. 2014;37(1):115-126.
- [Google Scholar]
- Craniovascular traits in anthropology and evolution: from bones to vessels. J Anthropol Sci. 2017;95:35-65.
- [Google Scholar]
- Emissary foramens of the human skull: anatomical characteristics and its relations with clinical neurosurgery. Int J Morphol. 2013;31(1):287-292.
- [Google Scholar]
- The size of selected human skull foramina in relation to skull capacity. Folia Morphol (Warsz). 2006;65(4):301-308.
- [Google Scholar]
- First febrile seizures. Characteristics of the child, the seizure, and the illness. Clin Pediatr (Phila). 1994;33(5):263-267.
- [Google Scholar]
- Febrile seizure: demographic features and causative factors. Iran J Child Neurol. 2012;6(4):33-37.
- [Google Scholar]
- Febrile seizures: factors affecting risk of recurrence in Pakistani children presenting at the Aga Khan University Hospital. J Pak Med Assoc. 2003;53(1):11-17.
- [Google Scholar]
- Risk factors of the first febrile seizures in Iranian children. Int J Pediatr. 2010;2010:862897.
- [Google Scholar]
- The influence of vaccine on febrile seizure. Curr Neuropharmacol. 2018;16(1):59-65.
- [Google Scholar]
- Vaccines and febrile seizures: quantifying the risk. Pediatrics. 2016;138(1):e20160976.
- [Google Scholar]
- National trend survey of hospitalized patients with febrile seizure in the United States. Seizure. 2017;50:160-165.
- [Google Scholar]
- FEBSTAT Study Team. Distribution of febrile seizure duration and associations with development. Ann Neurol. 2011;70(1):93-100.
- [Google Scholar]
- Febrile seizures: guideline for the neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics 2011
- [CrossRef] [Google Scholar]
- Role of electroencephalogram and neuroimaging in first onset afebrile and complex febrile seizures in children from Kashmir. J Pediatr Neurosci. 2012;7(1):9-15.
- [Google Scholar]
- Results of computed tomography in “neurologically normal” children after initial onset of seizures. Pediatr Neurol. 1989;5(2):102-106.
- [Google Scholar]
- Risk of intracranial pathologic conditions requiring emergency intervention after a first complex febrile seizure episode among children. Pediatrics. 2006;117(2):304-308.
- [Google Scholar]






