Translate this page into:
Anxiety in Parkinson’s Disease: Correlation with Depression and Quality of Life
Birinder S. Paul, MD, DM Department of Neurology Dayanand Medical College and Hospital, Ludhiana, 141001, Punjab India drbirinder06@yahoo.co.in
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Introduction Anxiety is common in patients with Parkinson’s disease (PD). Its prevalence ranges from 20 to 40% but despite that, the high prevalence anxiety in PD is often undiagnosed and untreated. This research was aimed to study the pattern of anxiety with regard to its prevalence and risk factors and to establish the association of anxiety with depression and quality of life (QOL) in patients with PD.
Methods A total of 105 patients with PD were prospectively observed. Demographic and clinical variables were recorded and patients were assessed for anxiety (the Parkinson anxiety scale [PAS]), depression (geriatric depression scale [GDS]), and QOL (Parkinson’s Disease Questionnaire-39 [PDQ-39]). Multiple forward logistic regression analysis was done for parameters showing association with anxiety. Pearson’s correlation was used to calculate the strength of association of depression and QOL with anxiety.
Results Anxiety was present in 56 PD patients (53.3%). Episodic anxiety was noted in 50%, avoidance behavior in 35%, and persistent anxiety in 15% of these patients. There was significant association of anxiety with duration of disease (p = 0.001), severity (p < 0.005), levodopa equivalent dose (LED; p = 0.001), and tremor phenotype of PD (p = 0.004). Anxiety coexisted with depression in 50 patients (79.4%), which was statistically significant in our cohort (p = 0.001). There was significant linear relationship between the PAS and PDQ-39.
Conclusion Anxiety exerts a negative impact on the QOL as revealed by proportionately worsening PDQ-39 and PAS scores. Screening for anxiety will allow efficient delivery of support and treatment to patients with PD and their families.
Keywords
anxiety
Parkinson’s anxiety scale
quality of life
anxiety and depression
Parkinson’s disease
Introduction
Anxiety is a common no-motor symptom noted in patients with Parkinson’s disease (PD). Its prevalence is heterogeneous and complex. Anxiety has a significant impact on caregiver burden, treatment compliance, cognition, and can also increase risk of falls.1 It has been shown that number of PD patients who experience anxiety is larger than other chronic neurological disorders such as multiple sclerosis or epilepsy.2 Anxiety is more than just a symptom, rather anxiety in PD presents with wide range of symptoms, probably originating from a central cause. Impact of anxiety on quality of life (QOL) appears to be more than the motor symptoms of PD.1 The interaction between the motor symptoms of PD and anxiety often result in a vicious cycle where anticipation of the motor symptoms triggers anxious behavior and vice versa.3 There is lack of data regarding anxiety and its association with QOL, especially in PD patients. Research on PD-specific anxiety and its risk factors is need of the hour to determine whether it significantly affects QOL and facilitates appropriate treatment of such patients. The prevalence rate of anxiety varies widely (20–46%), though extreme values up to 73% have been reported.1 4 This wide range of variability in prevalence is due to heterogeneity in the scales assessing anxiety, challenges in identifying type of anxiety, and overlapping symptomatology which include depression. Thus, to overcome the limitations of the preexisting scales, the International Consortium in 2011 developed a new scale, the Parkinson anxiety scale (PAS), for assessing anxiety symptoms in PD.5
Given the fact that anxiety in PD is under recognized and also has profound effect on living, the present study aimed to study pattern of anxiety with regard to its prevalence in PD, its risk factors, and to establish the association of anxiety with depression and QOL.
Materials and Methods
This was a prospective observational hospital-based study conducted in a tertiary care health center. All adult patients of idiopathic PD, diagnosed as per Movement Disorder Society (MDS),6 attending the neurology clinic during their regular outpatient visit were included. Informed consent was taken from the patients in their vernacular language and the study was approved by the Institutional Ethical Committee. PD patients with dementia (Mini-mental state examination (MMSE) < 23) and secondary Parkinsonism were excluded. PD patients who were already on anxiolytic or antidepressants medications were also excluded.
Data Collection and Analysis
Demographic, clinical variables, and PD-specific factors recorded on a predesigned structured proforma included age, gender, duration of disease, severity (Hoehn and Yahr staging scale [H&Y]), and unified PD rating scale (UPDRS III) score. The patients were further categorized as akinetic/rigid PD (AR) or tremor PD (T) phenotypes, using the modified ratio developed by Schiess et al.7 Calculation of levodopa equivalent dose (LED) in milligrams was done as per the conversion factor of individual drugs.
Participants were informed that the aim of the study was to assess anxiety and its association with disease factors and QOL. Patients and their informants were assessed with a proforma that included questionnaires, namely, PAS, geriatric depression scale (GDS), and Parkinson’s Disease Questionnaire-39 (PDQ-39) for assessing QOL.
Anxiety Scale
PAS is a comprehensive scale which reliably measures the anxiety symptoms capturing its multiple dimensions. PAS accesses the following three characteristic anxiety domains: (1) persisting anxiety (five items), (2) episodic anxiety (four items), and (3) avoidance behavior. There are 12 items, with each item being scored on 5-point Likert’s scale; “0” on the scale means “never” and “4” means “severe,” the maximum total score being 48. The scale was translated into Punjabi and Hindi for the purpose of this study. Following vernacular translation by language experts, this was translated back to English and checked for inconsistencies. It was then administered to 10 PD patients and 10 controls, in the language of their choice to see the reliability and feasibility of the scale. The value of >13 was taken as cut-off for the presence of anxiety in PD patients.8 Also the most optimal cut-off value for the three subscales were defined as 9.5 for subscale for persistent anxiety (generalized anxiety disorder), 4.5 for subscale for episodic anxiety (panic disorder), and 3.5 for subscale for avoidance behavior (avoidance anxiety disorder) to identify the subtype of anxiety associated with PD in our cohort.8
Depression Scale
GDS is a self-assessment scale used for assessing depression. The patients report the 30 items of the scale as “yes/no” with respect to their experience of their symptoms. Each answer as “yes” scores one mark with maximum score of 30. Patients with score of more than 10 on GDS were considered as having depression.9
This scale is a screening tool recommended by MDS to identify depression among PD patients. It not only has good internal consistency but also reliable results on test–retest. It is highly correlated with Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) for diagnosis of depression in PD and is easy to administer.10
Quality of Life
PDQ-39 is a disease specific 39-item questionnaire that was used to assess QOL. It assesses eight dimensions of daily living that reflect the subjective feeling of well being, motivation, satisfaction, functioning, and impairments. The final score is expressed by adding the value of individual items and expressing as percentage. Lower score on PDQ-39 indicates good QOL.
Analysis of Data
The collected data were statistically analyzed using software SPSS version 20.0. Mean and standard deviations were computed and Chi-square and Z-tests were applied for proportion. Prevalence of anxiety was calculated as percentage. Multiple forward logistic regression analysis was done for parameters associated with anxiety. QOL was assessed by calculating the summary index of PDQ-39. The Z-score was calculated for all disease variables. Pearson’s correlation was then used to calculate strength of association between variables and PDQ-39. A p-value of ˂0.05 was considered as significant. Correlation coefficient ˃0.5 was considered significant, the greater the value than 0.5, the stronger the correlation.
Results
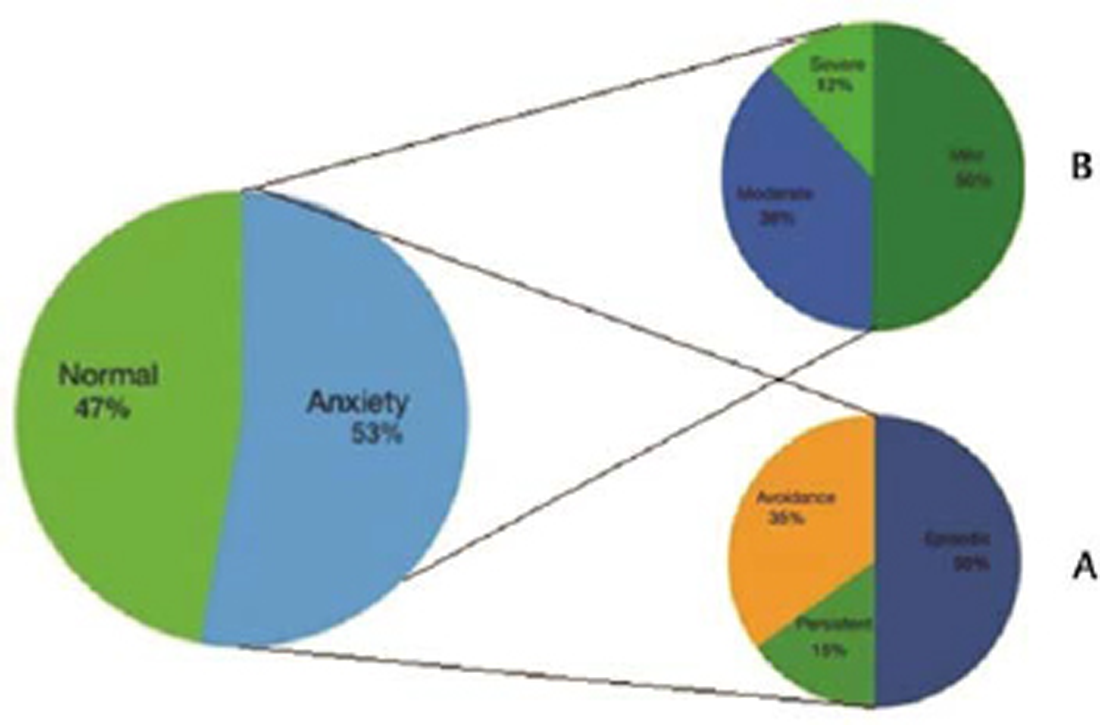
A total of 105 patients with PD who fulfilled the inclusion criteria from the neurology outpatient were included. There were 79 males (75.2%) and 26 females (24.7%) and the mean age of these 105 patients was 61.2 + 10.4 years. The mean age of onset and H&Y stage was 57.5 + 9.6 and 2.2 + 0.9 years, respectively. The mean duration of disease was 3.8 + 3.5 years. In our cohort, 58 (54.7%) patients had tremor phenotype of PD, while 47(45.3%) had the rigid phenotype. The mean UPDRS score was 53.5 + 15.4 and mean LED was 449.6 + 169.9 mg. Based on the cut-off value of >13 on the PAS scale, anxiety was present in 56 (53.3%) patients with the mean PAS score of 16.7 + 11.1. To study the spectrum of anxiety in our cohort of PD patients scoring for each subscale of PAS was also applied. The subtype of anxiety observed in decreasing order of frequency was episodic anxiety (50%), avoidance behavior (35%), and persistent anxiety (15%; Fig. 1).
-
Fig. 1 Anxiety spectrum prevalent in patients with Parkinson’s disease. (A) Distribution according to subdomains. (B) Distribution according to severity.
Fig. 1 Anxiety spectrum prevalent in patients with Parkinson’s disease. (A) Distribution according to subdomains. (B) Distribution according to severity.
There was significant association of anxiety with duration (p = 0.001), severity of disease (p < 0.005), and LED (p = 0.001). In addition, there was direct relation of anxiety with tremor phenotype, which demonstrated statistically significant higher score (20.54 ± 11.64), as compared with rigid phenotype (12.21 ± 8.62). In our cohort, other demographic factors, like age, age of onset, gender, and educational status, were not different between patients with or without anxiety (Table 1).
|
Characteristic |
Normal (n = 49) |
Anxiety (n = 56) |
Z-value |
p-Value |
|---|---|---|---|---|
|
Abbreviations: GDS, geriatric depression scale; H&Y, Hoehn and Yahr staging scale; LED, levodopa equivalent dose; PD, Parkinson’s disease; UPDRS, unified PD rating scale. |
||||
|
Age (y) |
60.23 + 10.52 |
66.64 +9.19 |
0.728 |
0.116 |
|
Duration (y) |
2.22 + 2.07 |
6.17 +4.38 |
3.973 |
0.001 |
|
Age of onset (y) |
58.10 + 9.84 |
60.57 +8.35 |
0.340 |
0.137 |
|
H&Y |
1.83 + 0.74 |
2.61 +0.74 |
3.685 |
0.000 |
|
UPDRS |
32.06 + 15.14 |
86.50+17.77 |
5.601 |
<0.001 |
|
LED |
352.09 + 134.21 |
564.61+174.98 |
4.738 |
<0.001 |
|
GDS |
6.97 + 3.05 |
17.93 +5.31 |
5.192 |
<0.001 |
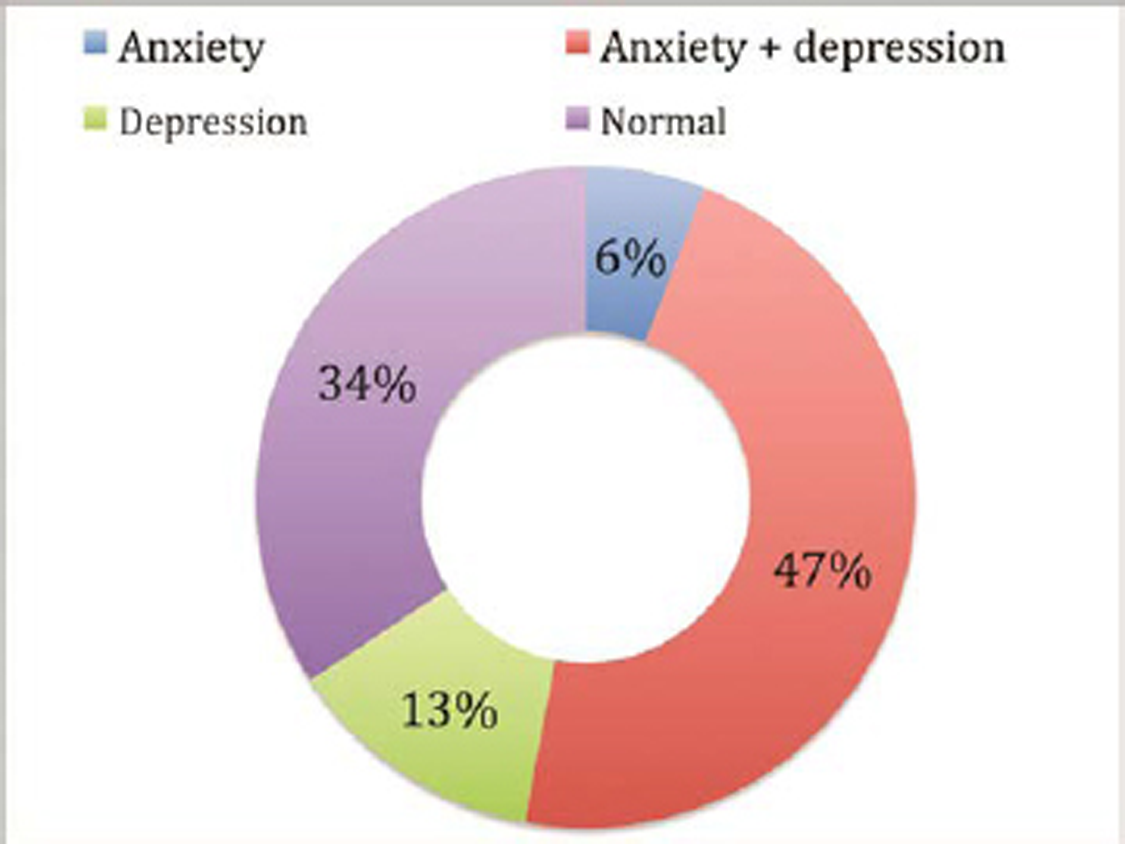
Our study shows that anxiety coexisted with depression in 50 patients (79.4%), while depression alone was noted in 13 (20.6%) and anxiety alone in only 6 patients (9.5%), as shown in Fig. 2. There was statistically significant correlation (p = 0.001) of coexistence of anxiety and depression.
-
Fig. 2 Distribution of anxiety and depression prevalent in patients with Parkinson’s disease (n = 105).
Fig. 2 Distribution of anxiety and depression prevalent in patients with Parkinson’s disease (n = 105).
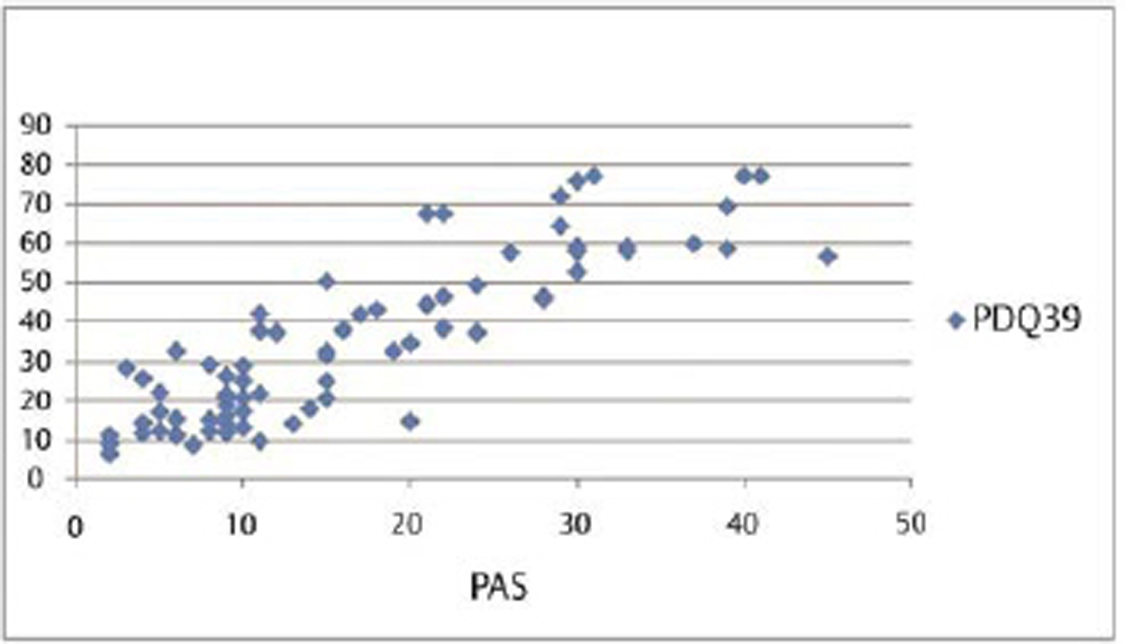
Our results show that although duration, severity of disease, UPDRS, and LED had association with PDQ-39, however, QOL index PDQ-39 had significant correlation both with anxiety and depression (Table 2). Also, anxiety showed the maximum Pearson’s correlation value (0.871) with PDQ-39. Partial regression plot shows that as severity of anxiety worsens, the QOL is revealing a direct linear relationship between the PAS and PDQ-39 (Fig. 3
-
Fig. 3 Impact of anxiety assessed by PAS on quality of life assessed by PDQ-39 represented as partial regression plot and bar diagram. PAS, the Parkinson anxiety scale.
Fig. 3 Impact of anxiety assessed by PAS on quality of life assessed by PDQ-39 represented as partial regression plot and bar diagram. PAS, the Parkinson anxiety scale.
|
Characteristics |
Duration |
H&Y |
UPDRS |
GDS |
PAS |
PDQ-39 |
|
|---|---|---|---|---|---|---|---|
|
Abbreviations: GDS, geriatric depression scale; H&Y, Hoehn and Yahr staging scale; LED, levodopa equivalent dose; PAS, the Parkinson anxiety scale; PD, Parkinson’s disease; UPDRS, unified PD rating scale. |
|||||||
|
Duration |
Pearson’s correlation |
– |
– |
– |
– |
– |
– |
|
H&Y |
Pearson’s correlation |
0.331 |
– |
– |
– |
– |
– |
|
UPDRS |
Pearson’s correlation |
0.457 |
0.595 |
– |
– |
– |
– |
|
GDS |
Pearson’s correlation |
0.315 |
0.299 |
0.695 |
– |
– |
– |
|
PAS |
Pearson’s correlation |
0.419 |
0.459 |
0.772 |
0.733 |
– |
– |
|
PDQ-39 |
Pearson’s correlation |
0.454 |
0.503 |
0.836 |
0.769 |
0.871 |
– |
|
LED |
Pearson’s correlation |
0.448 |
0.468 |
0.630 |
0.510 |
0.582 |
0.644 |
Discussion
Our study shows that anxiety is present in 53.3% patients of PD with episodic anxiety as the most common subtype. Disease severity, duration, and tremor subtype are some of the risk factors of anxiety in our study. Lastly, anxiety in PD can coexist with depression and has a significant negative impact on QOL, even more than depression. This the first study from India regarding risk factors causing anxiety in PD patients and its effect on the life of these patients.
Anxiety prevails in every stage of disease and has been ranked as one of the three most unmet disabilities of these patients.11 Anxiety has significant impact on life, more than the motor symptoms; however, it remains commonly undiagnosed and untreated. Studies in literature have reported that prevalence of anxiety varies from 20 to 70% among different ethnic populations revealing the fact that it is even more prevalent than depression in PD population.12 The prevalence of anxiety in this study was 53.3%, which is higher as compared with previous studies (30–40%), which may be contributed by the social–cultural factors prevalent in Indian PD population. Another reason may be the use of a highly validated screening tool in the vernacular language that led to detection of comprehensive anxiety symptoms including avoidance behavior, which itself contributed to 35% of anxiety in our group of patients. Avoidance subtype represents behavioral symptom highlighting that anxiety is not just a reaction to physical motor symptoms but itself a nonmotor symptom of PD.
Broen et al described PD-specific anxiety based on DSM-IV diagnostic criteria in decreasing order of frequency as generalized anxiety (14–20%), social fear (13.8%), and not specified anxiety symptoms (13.3%).4 But anxiety symptoms, especially in PD patients, need a comprehensive assessment scale which assesses all aspects of anxiety. The previous scales were biased toward detecting persistent symptoms as noted in the Hamilton anxiety rating scale (HARS) or episodic symptoms as in the Beck anxiety inventory (BAI).13 The other available scales do not evaluate avoidance behavior of anxiety. The PAS used in this study was specifically developed for PD patients with three subscales that address all aspects of the anxiety.
Our results distinctly show that episodic anxiety more uniquely associated with PD. There are different pathophysiological pathways and neurotransmitters involved in symptomatology of subscales of anxiety.14 This higher prevalence of episodic anxiety may be postulated to be a PD-specific anxiety symptom related to dopaminergic pathway as suggested by Weintraub et al.15 In addition, other pathophysiological reasons, such as loss of catecholaminergic cells and abnormalities of serotonin production, also explain avoidance behavior of anxiety and persistent anxiety symptoms in PD that do not improve with dopaminergic treatment.16 These two domains together contributed to approximately 50% of anxiety symptoms in our study, which may also be the reason for higher mean LED (p = 0.000) dose seen in the group of patients.
PD-specific risk factors for anxiety in our study were disease duration, motor symptoms, H&Y stage, and LED. Similarly, Dissanayaka et al in their studies showed that disease severity, dyskinesia, and motor fluctuations were PD-specific risk factors for anxiety.17 This association of disease factors may be related to degeneration of dopaminergic pathway that occurs with time which possibly sets up the vicious circle of worsening of motor symptoms, requiring higher levodopa dose leading to worsening severity of disease resulting in evoking more anxiety. Functional imaging study by Picillo et al showed correlation between anxiety and dopaminergic dysfunction in newly diagnosed drug naïve PD patients.18 They also demonstrated that low dopamine uptake in the caudate nucleus was associated with anxiety traits confirming that dopamine dysfunction is responsible for anxiety symptoms. Anxiety is also considered as an important nonmotor symptom, which directly correlates with the disease duration and severity thus supporting to be an epiphenomenon because of widespread neurodegenerative process in the brain.
First, we observed tremor phenotype of disease as a significant risk factor for anxiety and this has not been previously reported. This may be a reaction to the visible motor symptom of shaking hands resulting in behavior changes in which motor symptom triggers anxiety. Second, tremors are generally less responsive to dopaminergic treatment highlighting the widespread degenerative process involving the nondopaminergic cerebellothalamocortical pathways in tremor phenotypes of PD.19 Evidence in literature shows significant association of disease duration and levodopa dose in PD with anxiety, though they have used different anxiety rating scales. Higginson et al reported that there is reduction in anxiety behavior after surgical treatment of PD. This reduction in anxiety highlights that anxiety symptoms are more disease specific and not a reaction to motor symptoms.20
The results of our study show that approximately 60% participants had associated depression. Coexistence of anxiety and depression was seen in almost 80% patients and anxiety alone was seen in only 9.5%. Cui et al also showed that 86.6% of patients’ depression was coexisted with anxiety and 1.5% patients had only depression without anxiety.21 This supports the fact that both anxiety and depression are core features and are risk factors for each other. Hence, an anxious-depressive subtype exists in PD which is supported by this and our study also. Both anxiety and depression show common pathophysiological mechanism of dopaminergic dysfunction. However, they can be present in PD patients in the absence of each other also.22
In PD patients, the QOL is poor as compared with general population. Studies regarding factors affecting QOL have variable and conflicting results. Schrag et al had shown that disease duration, severity, and gait disturbances were the risk factors impacting life of PD patients.23 On the other hand, another study found cognitive status as assessed by mini mental status being the strongest predictor of QOL rather than the motor symptoms.24 In addition, mood disorders, especially depression, were also documented as the risk factor for poor QOL.
On the contrary, some previous studies have reported that depression has more impact on QOL in PD. This may be because both anxiety and depression were not included in their model of evaluation of PD patients and QOL.19 Some investigators have suggested that anxious personality in itself leads to development of PD in later life, signifying degeneration of dopaminergic pathways is common physiological change noted in both anxiety and PD. Also, it has been noted that often anxiety is present before the onset of depressive symptoms. In the current study, depression and motor severity were also found to have influence the QOL but anxiety was the strongest factor affecting QOL with Pearson’s correlation of 0.871.
Strengths and Limitations
The strength of this study is that it was prospective observational study including 105 patients with PD. We used validated PAS scale for assessing all dimensions of anxiety. The study also adequately addressed the frequency of episodic anxiety along with other subtype of anxiety in PD.
Limitations of this study are that anxiety was not assessed separately during off-periods and UPDRS-IV score was not included in the analysis. PD is considered as noncurable progressive disorder and disease-related apprehension, fear, uncertainty, and phobia may be contributory factors for anxiety. These cannot be ruled out, although we used self-reported scales validated in the patient’s vernacular language for rating anxiety and depression. Future research taking into account these limitations may improve our understanding regarding physiology of anxiety and preventive strategies to improve life of these patients.
Conclusion
In conclusion, anxiety is common in PD with episodic anxiety being the most common subtype. Disease factors, namely, severity, duration, and LED are significantly associated with anxiety. An “anxious-depressive” subtype exists in PD having a significant negative impact on QOL. Screening for anxiety may allow efficient delivery of support and treatment to patients and their families. Early identification of anxiety would prevent the onset of secondary depression and improve the QOL.
Conflict of Interest
None declared.
Funding None.
References
- The clinical spectrum of anxiety in Parkinson’s disease. Mov Disord. 2014;29(8):967-975.
- [Google Scholar]
- Impact of anxiety on quality of life in Parkinson’s disease. Parkinsons Dis. 2012;2012:640-707.
- [Google Scholar]
- Clinical presentation of anxiety in Parkinson’s disease: a scoping review. OTJR (Thorofare, NJ). 2016;36(3):134-147.
- [Google Scholar]
- Prevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2016;31(8):1125-1133.
- [Google Scholar]
- The Parkinson anxiety scale (PAS): development and validation of a new anxiety scale. Mov Disord. 2014;29(8):1035-1043.
- [Google Scholar]
- MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-1601.
- [Google Scholar]
- Parkinson’s disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat Disord. 2000;6(2):69-76.
- [Google Scholar]
- Prevalence of anxiety among Hungarian subjects with Parkinson’s disease. Behav Neurol. 2017;2017:1470149.
- [Google Scholar]
- Depression rating scales in Parkinson’s disease: a critical review updating recent literature. J Affect Disord. 2015;184:216-224.
- [Google Scholar]
- Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2007;22(8):1077-1092.
- [Google Scholar]
- Priority setting partnership to identify the top 10 research priorities for the management of Parkinson’s disease. BMJ Open. 2014;4(12):e006434.
- [Google Scholar]
- Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Mov Disord. 2000;15(4):669-677.
- [Google Scholar]
- Validity and reliability of the geriatric anxiety inventory in Parkinson’s disease. Australas J Ageing. 2012;31(1):13-16.
- [Google Scholar]
- Predictors of anxiety in early-stage Parkinson’s disease - results from the first two years of a prospective cohort study. Parkinsonism Relat Disord. 2017;43:49-55.
- [Google Scholar]
- Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson’s disease. J Nucl Med. 2005;46(2):227-232.
- [Google Scholar]
- Involvement of prelimbic 5-HT7 receptors in the regulation of anxiety-like behaviors in hemiparkinsonian rats. Neurol Res. 2018;40(10):847-855.
- [Google Scholar]
- Anxiety disorders in Parkinson’s disease: prevalence and risk factors. Mov Disord. 2010;25(7):838-845.
- [Google Scholar]
- Association between dopaminergic dysfunction and anxiety in de novo Parkinson’s disease. Parkinsonism Relat Disord. 2017;37:106-110.
- [Google Scholar]
- Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson’s disease. Neuroscience. 2011;177:230-239.
- [Google Scholar]
- Which symptoms of anxiety diminish after surgical interventions for Parkinson disease? Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(2):117-121.
- [Google Scholar]
- Prevalence and risk factors for depression and anxiety in Chinese patients with Parkinson disease. BMC Geriatr. 2017;17(1):270.
- [Google Scholar]
- Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314-1322. (pt 6)
- [Google Scholar]
- How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15(6):1112-1118.
- [Google Scholar]
- Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord. 2002;17(1):60-67.
- [Google Scholar]






