Translate this page into:
Alleviating Stress Response to Tracheal Extubation in Neurosurgical Patients: A Comparative Study of Two Infusion Doses of Dexmedetomidine
Address for correspondence: Dr. Ankur Luthra, H. No. 979, Aashirwad Enclave, Sector 49-A, Chandigarh - 160 047, India. E-mail: zazzydude979@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Tracheal extubation is almost always associated with increase in sympathoadrenal activity may result in hypertension, tachycardia, and arrhythmias. Attempts have been made to oppose the pressor response by the use of various drugs. Dexmedetomidine decreases norepinephrine which reduces the blood pressure and the heart rate (HR). We hypothesize that the infusion of dexmedetomidine may produce more stable hemodynamics during extubation as compared to boluses.
Materials and Methods:
Ninety adult patients aged 18–65 years, the American Society of Anesthesiologists Grade I–II undergoing intracranial surgeries for various neurologic problem at All India Institute of Medical Sciences were enrolled in this randomized controlled trial. Primary
Objectives:
(1) To observe the hemodynamic changes (HR and mean arterial pressure [MAP]) and airway responses during tracheal extubation following two infusion doses of dexmedetomidine.
Secondary Objectives:
(1) Time to emergence and time to tracheal extubation, (2) Early postoperative complications such as laryngospasm and bronchospasm, and (3) adverse effects of the study drug. Patients were assigned into three groups – (1) Group D0.2 – 0.2 μg/kg/h diluted to 50 ml, (2) Group D0.4 – 0.4 μg/kg/h diluted to 50 ml and Group P (Placebo) – 0.9% NS 50 ml. The hemodynamics including the HR and MAP were recorded just before the loading dose of the study drug and then were recorded every 5 min till the infusion was stopped at tracheal extubation and every 1 min till 10 min postextubation. In addition, the airway, respiratory and cardiovascular complications along with postoperative nausea and vomiting, shivering, cough grading, Aldrete score, Ramsay sedation scale, and intraoperative awareness were recorded.
Statistical Analysis:
Continuous variables such as HR and MAP were analyzed using analysis of variance and categorical variables were analyzed using the Chi-square test.
Results:
Patient demographics were comparable between the three groups. There was a significant reduction in HR and MAP just before extubation and up to 10 min post extubation in the D0.2 and D0.4 groups as compared to placebo (P < 0.001) but the difference among the dexmedetomidine groups were not significant. Patients belonging to D0.2 group emerged faster than D0.4 group, however, the results were comparable with placebo group. 73.3% patients of the placebo group had tachycardia and hypertension at emergence as compared to only 3.3% patients in the D0.4 group (P < 0.001). Eighty percentage patients of D0.2 and 100% patients of D0.4 group had a significant reduction in cough as compared to placebo (P < 0.001). No patient in either groups had intraoperative awareness, any respiratory complications, or allergic reactions to the study drug. Modified Aldrete scoring and Ramsay sedation scale were comparable in all the three groups.
Conclusion:
Dexmedetomidine suppresses cough and hemodynamic responses (HR and MAP) to tracheal extubation significantly without delaying emergence.
Keywords
Comparative
dexmedetomidine
extubation
infusion
neurosurgical
stress
INTRODUCTION
Intracranial surgery requires the method of anesthesia that ensures hemodynamic stability and fast recovery to allow immediate neurological evaluation. Tracheal extubation is always associated with hemodynamic changes because of sympathetic discharge due to epipharyngeal and laryngeal stimulation.[1] This increase in sympathetic activity may result in hypertension, tachycardia, and arrhythmias.[12] The increase in blood pressure (BP) and heart rate (HR) is transitory, variable but unpredictable and is more hazardous to the patient with preexisting hypertension, myocardial insufficiency, or cerebrovascular diseases.[3] Hypertension can result in increase of brain edema or intracranial hematoma formation which may give rise to herniation. The occurrence of hypertension during emergence has been reported to be more than 90% in neurosurgical patients.[45] Thus, to prevent and control the hemodynamic response to nociceptive stimuli is of utmost importance to preserve cerebral homeostasis in these patients. Airway irritation appearing during tracheal extubation may cause cough or difficulties in breathing. Smooth extubation requires the absence of straining, movement, coughing, breath holding, or laryngospasm.[6]
Various techniques and antihypertensive drugs are available to reduce airway and circulatory responses during extubation, but none have been completely successful.[56789] Attempts have been made to oppose the hemodynamic response by the use of drugs such as narcotic analgesics,[7] deepen the plane of anesthesia by inhalation agents,[6] vasodilator agents,[8] local anesthetics[910] and adrenoceptor blockers. Studies have been carried out with the use of lignocaine,[2910] esmolol,[51112] nicardipine,[13] labetalol,[5] diltiazem,[123] opioids,[14] clonidine[15] as single dose, or in comparison with each other. Among the anesthetics, propofol has been shown to be very effective in attenuating the sympathetic response to tracheal extubation in cardiac patients.[1617]
Dexmedetomidine, a potent, highly selective alpha-2 receptor agonist that has sedative, analgesic, anxiolytic, and amnesic effects without causing significant respiratory depression.[18] It also has a sympatholytic effect, similar to clonidine, causing decrease in the concentration of norepinephrine. This in turn decreases the BP and the HR. It may thus be theoretically appropriate for reducing airway and circulatory reflexes during emergence from anesthesia. It has also been studied as been given as single bolus dose to attenuate airway, circulatory reflexes, and recovery responses during tracheal extubation.[1819] According to the mathematical model of drug pharmacokinetics, infusion of a drug at a constant rate produces a steady-state drug concentration which is more uniform than the bolus administration of the same drug. Thus, we hypothesize that the infusion of dexmedetomidine may produce more stable hemodynamics during tracheal extubation as compared to boluses.
Aims and objectives
Primary objectives
-
To observe the hemodynamic changes (HR and mean arterial pressure [MAP]) during tracheal extubation following two infusion doses of dexmedetomidine
-
To observe airway response during tracheal extubation following two infusion doses of dexmedetomidine.
Secondary objectives
-
Time to emergence and time to tracheal extubation
-
Early postoperative complications such as laryngospasm, bronchospasm, etc.
-
Adverse effects of the study drug, if any, such as delayed arousal, respiratory depression, and bradycardia.
MATERIALS AND METHODS
After taking approval from the Institute Ethics Committee (IRB No. IESC/T-188/04.05.2012) by Dr. Rakesh Yadav (Chairperson, Ethics Committee, All India Institute of Medical Sciences, New Delhi,) on May 7, 2012, 90 adult patients with the American Society of Anesthesiologists (ASA) physical Status I–II, aged between 18 and 65 years undergoing elective intracranial surgeries such as brain tumor and epilepsy surgeries who were likely to get tracheally extubated at the end of surgery were included in the study.
Exclusion criteria
The following criteria were considered for excluding the patients from enrollment - patients with associated cardiac, renal, hepatic and respiratory dysfunction, those on β-blockers, digoxin, α2-agonists, or psychotropic medications, or with HR below 60/min or arterial pressure <100/60 mmHg, or those with a history of allergic reactions to study drug. The patients who required postoperative mechanical ventilation, those with difficult airway or having second- or third-degree atrioventricular blocks and pregnant/nursing woman or morbidly obese patients were also excluded from the study.
Information pertinent to the preoperative history, physical examination, investigations was recorded. Demographic data (age, sex, weight, ASA status, and diagnosis) were noted. After the standard fasting protocol, these patients were premedicated with glycopyrrolate 0.2 mg intramuscular, before shifting them to the operation theater. Monitoring in the operation theater included an electrocardiogram, pulse oximeter, systolic, and diastolic arterial BP.
General anesthesia was induced with fentanyl 2 μg/kg followed by propofol 1.5–2 mg/kg and rocuronium 1 mg/kg to facilitate tracheal intubation. All patients were mechanically ventilated at a fresh gas flow of 2 L/min to maintain an end-tidal CO2 of 32 ± 2 mm Hg. Anesthesia was maintained with sevoflurane in 60% nitrous oxide and 40% oxygen mixture targeting a minimum alveolar concentration value of 0.8–1.2. Vecuronium in a dose of 0.02 mg/kg was given every 30–40 min for muscle relaxation. Arterial pressure was monitored using a 20 G cannula placed in the left or right radial artery. The values for HR, systolic, diastolic, and mean BP obtained just before the administration of study drug was considered baseline. Both groups received fentanyl 1 μg/kg and for an increase in HR or systolic BP 20% above the preincisional value sustained for 1 min. HR <50 beats/min sustained for 1 min was defined as bradycardia and atropine 0.6 mg was given to treat it. Systolic arterial pressure <20% from baseline sustained for 1 min was defined as hypotension which was treated with fluid bolus or mephentermine 6 mg. A computer-generated randomization chart was used, and patients were allocated to different groups using a sealed opaque envelope method. The three study groups were, Group D0.2 – dexmedetomidine 0.2 μg/kg/h infusion diluted to 50 ml, Group D0.4 – dexmedetomidine 0.4 μg/kg/h infusion diluted to 50 ml, and Group P (Placebo) – 0.9% normal saline 50 ml.
All patients in the intervention groups D0.2 and D0.4 received a loading dose of dexmedetomidine 0.5 μg/kg diluted to 10 ml before the start of infusion over a period of 10 min, at the beginning of dural closure. The patients in Group P received an equivalent volume of saline both as loading and infusion doses. These infusions continued up till tracheal extubation. Study drug infusions were prepared by varying the drug dilution of dexmedetomidine in the 50 ml syringe according to the patient's body weight by an anesthesiologist who was a part of the study but not involved in the anesthetic management or assessment of the patient. All the infusions ran at a standard rate of 4 ml/h. Thus, the attending anesthesiologist was blinded to the study drug concentration and the actual rate of infusion according to body weight of the patient. The hemodynamics including the HR and MAP were recorded just before the loading dose of the study drug and then were recorded every 5 min till the infusion was stopped at tracheal extubation. At the last skin suture, inhalation anesthetic was discontinued. On return of spontaneous efforts, nitrous oxide was discontinued, and neuromuscular block reversed with neostigmine 0.05 mg/kg and glycopyrrolate 0.01 mg/kg. The maximum rise of HR and MAP was noted during tracheal extubation. Hemodynamic variables were noted every minute for 10 min posttracheal extubation. Emergence and extubation times were noted which were defined as emergence time – time interval between discontinuing of anesthetic and patient following verbal commands and Extubation time – time interval between cessation of anesthetics and tracheal extubation. Delayed emergence was defined as emergence time more than 15 min after discontinuing anesthetics and reversal of neuromuscular blockade.
A decrease of SpO2 ≤92% was defined as desaturation and holding breath for 20 s or more as breath-holding any episode of laryngospasm, bronchospasm, or desaturation was recorded. Any other immediate postoperative complications which include bradycardia, hypotension, postoperative nausea and vomiting (PONV), and restlessness were noted. Modified Aldrete Score was recorded for recovery in postanesthesia care unit and grading of cough[29] was noted as a measure for recovery response. Postoperative sedation was assessed using Ramsay sedation scale. A structured Brice interview[29] for detecting awareness under anesthesia was recorded from these patients 24 h postsurgery.
Statistical analysis
A total of 90 patients (30 in each group) were calculated at 80% power and Type-1 error of 0.05 using hemodynamics (HR and MAP) as primary outcome measure from a previous study done by Turan et al.[19] on dexmedetomidine used in intracranial surgeries.
Statistical analysis was carried out using STATA 11.2, (StataCorp LLC, Texas, USA). The demographic variables such as age, weight, patient position, ASA status, duration of surgery, and anesthesia and emergence time were analyzed using one-way analysis of variance after the Bonferroni correction. Continuous variables such as hemodynamics (HR and MAP) and categorical variables such as cardiovascular and respiratory complications, PONV, and shivering were analyzed using the Chi-square and Fisher's exact tests. Ramsay sedation scale was analyzed using the Kruce Wallis equality of proportions. P < 0.05 was considered statistically significant.
RESULTS
Ninety adult patients who were initially screened were enrolled in this study. There were no dropouts. The demographic details have been listed in Table 1 and perioperative and recovery characteristics in Table 2. The period of study drug infusion varied in the three groups which was longer in the placebo group as compared to the other two dexmedetomidine groups (P = 0.002), probably due to longer closure time in the placebo group. The emergence time in D0.4 group patients was 11.4 ± 4.1 min which was longer than the patients in the D0.2 group where emergence time was 8.2 ± 2.6 min (P < 0.0001). However, emergence time between the D0.2 and placebo group was comparable.
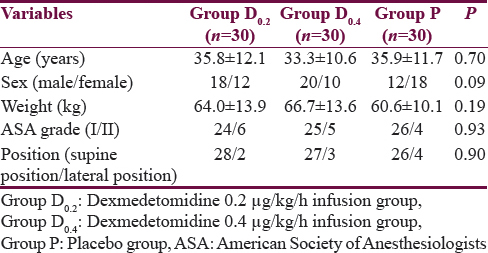
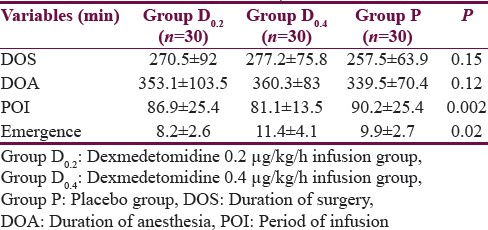
Hemodynamics
The perioperative trend of HR and MAP has been graphically shown in Figures 1 and 2, respectively. The HR and MAP values were higher in placebo group as compared to D0.2 and D0.4 groups, especially at the time of tracheal extubation.
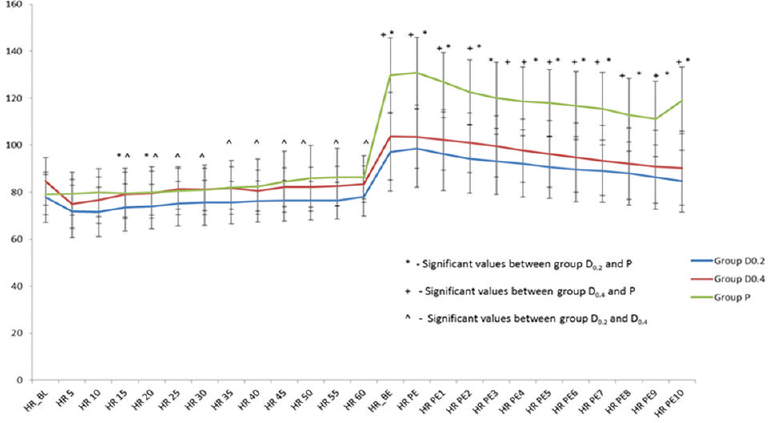
- Perioperative trend of heart rate in the 3 study groups expressed as mean standard deviation. HR: Heart rate, HR_BL: Baseline heart rate, HR 5, HR 10, HR 15, HR 20, HR 25, HR 30, HR 35, HR 40, HR 45, HR 50, HR 55, HR 60: Heart rates at 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 min from the start of drug administration, HR_BE: Heart rate just before tracheal extubation, HR PE: Heart rate immediately posttracheal extubation, HR PE1, HR PE2, HR PE3, HR PE4, HR PE5, HR PE6, HR PE7, HR PE8, HR PE9, HR PE10: Heart rates postextubation at 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 min, respectively, Group D0.2 – dexmedetomidine 0.2 μg/kg/h infusion group, Group D0.4 – dexmedetomidine 0.4 μg/kg/h infusion group, Group P – Placebo group
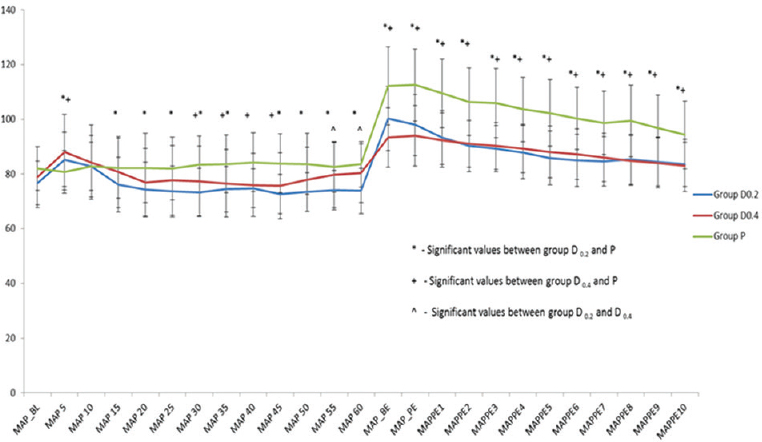
- Perioperative trend of mean arterial pressure in the 3 study groups expressed as Mean standard deviation. MAP: Mean arterial pressure, MAP_BL: Baseline MAP, MAP 5, MAP 10, MAP 15, MAP 20, MAP 25, MAP 30, MAP 35, MAP 40, MAP 45, MAP 50, MAP 55, MAP 60: Mean arterial pressures at 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 min from the start of drug administration, MAP_BE: MAP just before tracheal extubation, MAP PE: MAP immediately post tracheal extubation, MAPPE1, MAPPE2, MAPPE3, MAPPE4, MAPPE5, MAPPE6, MAPPE7, MAPPE8, MAPPE9, MAPPE10: MAP postextubation at 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 min, respectively, Group D0.2 – dexmedetomidine 0.2 μg/kg/h infusion group, Group D0.4 – dexmedetomidine 0.4 μg/kg/h infusion group, Group P – Placebo group
Cardiovascular complications
The cardiovascular complications such as emergence hypertension, tachycardia, and hypotension have been graphically shown in Figure 3. Around 73.3% patients in the placebo group suffered both hypertension and tachycardia during emergence, and 23.3% patients had emergence hypertension only; one patient in D0.4 group had emergence hypertension alone, and one patient had both hypertension and tachycardia. One patient in the D0.2 group had hypotension whereas no patient in this group had any episode of hypertension and tachycardia.
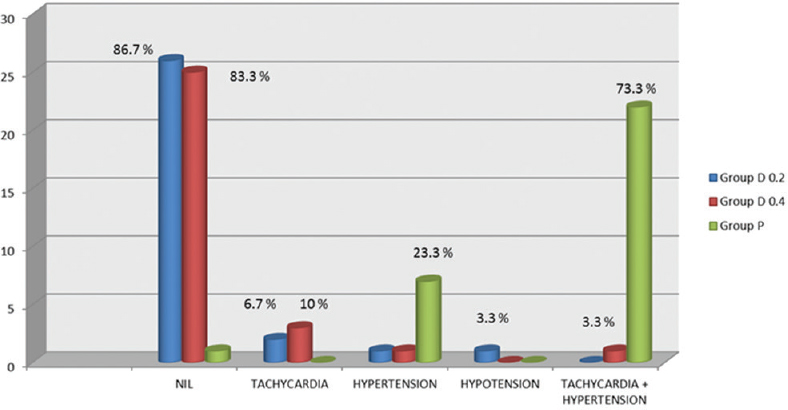
- Cardiovascular complications in the 3 study groups expressed as n (%). Group D0.2 – dexmedetomidine 0.2 μg/kg/h infusion group, Group D0.4 – dexmedetomidine 0.4 μg/kg/h infusion group, Group P – Placebo group
Delayed emergence
Six patients (20%) in Group D0.4 had delayed emergence as compared to 6.7% patients in placebo group. No patient in Group D0.2 had delayed emergence.
Postoperative nausea and vomiting and shivering
Five patients (16.7%) in placebo group had PONV, and two patients (6.7%) had shivering as compared to four (13.3%) patients in Group D0.4 who had PONV. None had shivering. No patient in Group D0.2 suffered PONV or shivering.
Cough
No patient coughed during emergence in Group D0.4 while in Group D0.2, absence of coughing was observed in 80% subjects. About 33.3% patients had mild cough in the placebo group whereas 20% patients had mild cough in Group D0.2. Around 40% and 3.3% patients in the placebo group had moderate and severe cough, respectively, as compared to dexmedetomidine group where no patient suffered moderate or severe cough [Figure 4].
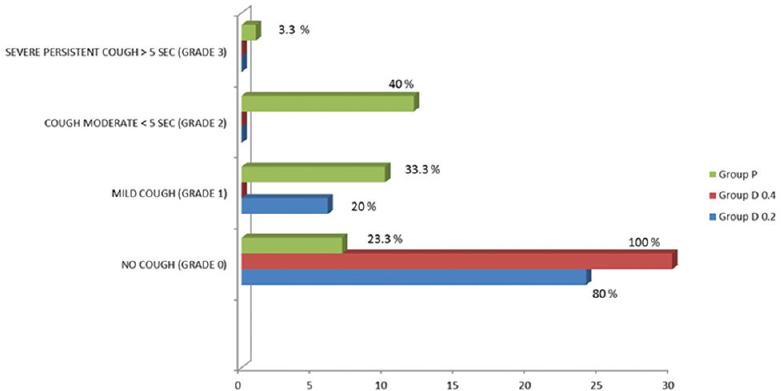
- Cough grade in patients in the 3 study groups expressed as n (%). Group D0.2 – dexmedetomidine 0.2 μg/kg/h infusion group, Group D0.4 – dexmedetomidine 0.4 μg/kg/h infusion group, Group P – Placebo group
No patient had respiratory complications, and none reported any intraoperative awareness. Twenty-five patients (83.3%) in the D0.4 group, 26 patients in Group D0.2 and 24 patients (80%) in the placebo group were cooperative and well oriented on emergence. Only one patient (3.3%) in Group D0.4 showed brisk response to stimulus (Ramsay sedation scale 4) and was deeply sedated. However, rest of the patients had a good recovery with an Aldrete score of 8–10 in the Intensive Care Unit, noted within 1 h postsurgery.
DISCUSSION
The hemodynamic responses to intracranial surgery are most often elicited at the beginning or at the end of the surgical procedure. These responses are transitory, variable, and unpredictable, more pronounced in hypertensive patients than in normotensive individuals. This hemodynamic stimulus is associated with increase in plasma adrenaline concentration parallel with the increase in BP.[23] The increase in adrenaline concentration during and after surgery may represent a physiological response to trauma and surgery. Transitory hypertension and tachycardia are probably of no consequence in healthy individuals, but either or both may be hazardous to those with hypertension, myocardial insufficiency, or cerebrovascular disease.
Our study demonstrates that dexmedetomidine in infusion doses of 0.2 and 0.4 μg/kg/h significantly alleviates hemodynamic responses to tracheal extubation without delaying emergence. In our study, there was a significant reduction in HR and MAP just before tracheal extubation and up to 10 min postextubation in both D0.2 and D0.4 groups (P < 0.001). Patients in D0.2 group had a faster emergence (8.2 ± 2.6 min), similar to the control group (9.9 ± 2.7 min) than those in D0.4 group (11.4 ± 4.1 min) though it was not clinically significant. There were no adverse reactions to the drug, no intraoperative awareness or any respiratory or airway complications. Modified Aldrete score and Ramsay sedation scale were also comparable in the three groups suggesting that dexmedetomidine could be safely used in neurosurgical patients without affecting the sensorium.
In neurosurgical patients, the use of agents that relax vascular smooth muscle to control the increase in arterial pressure at extubation should be avoided, as the resultant cerebral vasodilatation may increase cerebral blood volume and thus intracranial pressure. Various drugs which are alpha and beta adrenergic receptor antagonists have been advocated in such patients. Dexmedetomidine is a highly specific and selective α2-adrenergic agonist with sedative, anxiolytic, and analgesic effects.
Turan et al.[19] examined the effects of dexmedetomidine given at the end of procedure to prevent hyperdynamic responses during extubation and to allow a comfortable and high-quality recovery. Forty ASA I–III patients having elective intracranial surgery were included in the study. They found that dexmedetomidine 0.5 μg/kg administered 5 min before the end of surgery stabilized hemodynamics, allowed easy extubation, provided a more comfortable recovery, and early neurological examination following intracranial operations. Our findings are in accordance to the study by Turan et al.
Lawrence and De Lange[20] investigated the effect of a single preinduction intravenous dose of dexmedetomidine 2 μg/kg on anesthetic requirements and perioperative hemodynamic stability in 50 patients undergoing minor orthopedic and general surgery. Patients were anesthetized with nitrous oxide/oxygen/fentanyl, supplemented if necessary with isoflurane. The mean intraoperative isoflurane concentration was lower in the dexmedetomidine-treated patients than controls (0.01 [0.03]% compared to 0.1 [0.1]%; P = 0.001) although six of the 25 treated patients required isoflurane at some stage. The hemodynamic response to tracheal intubation and extubation was reduced in the dexmedetomidine group which was comparable to our study as was intraoperative HR variability; postoperative analgesic and antiemetic requirements, and perioperative serum catecholamine concentrations were lower in the dexmedetomidine group.
Guler et al.[21] studied the effect of a single bolus dose of dexmedetomidine 0.5 μg/kg as bolus intravenously over 60 s in comparison to a placebo in 60 ASA I–III patients undergoing intraocular surgery. Five minutes before the end of surgery, they were randomly allocated to receive either dexmedetomidine 0.5 μg/kg or saline intravenously over 60 s in a double-blind design. The blinded anesthetist awoke all the patients, and the number of coughs per patient was continuously monitored for 15 min after extubation; coughing was evaluated on a 4-point scale. Median coughing scores were 1 (1–3) in dexmedetomidine group and 2 (1–4) in placebo group (P < 0.05), but there were no differences between the groups in the incidence of breath holding or desaturation. The HR, systolic, and diastolic arterial pressures increased at extubation in both groups, but the increase was less significant with dexmedetomidine. The time from tracheal extubation and emergence from anesthesia were similar in both groups. Their findings suggested that a single bolus dose of dexmedetomidine before tracheal extubation attenuated cough and hemodynamic responses during extubation which was comparable to our study.
In 54 patients undergoing surgery for supratentorial brain tumors, Tanskanen et al.[24] demonstrated that the decrease in the hypertensive response to extubation was related to dexmedetomidine dose. Dexmedetomidine plasma target doses of 0.2 and 0.4 ng/ml were compared to placebo. Dexmedetomidine decreased the hemodynamic responses caused by stimuli during anesthesia; a higher dose was more effective than a lower dose which was in accordance with our study findings. In addition, the perioperative systemic infusion of dexmedetomidine at 1 μg/kg/h reduced patient-controlled epidural analgesia (PCEA) bolus consumption, prolonged the time for first request of rescue analgesia through the PCEA pump, and postoperative pain scores without severe side effects.
Our study also revealed that postextubation cough is effectively suppressed by both the infusion doses (0.2 and 0.4 μg/kg/h) of dexmedetomidine which was significantly lower than the control group. This may be due to the sedative action of dexmedetomidine and also because it relaxes the bronchial smooth muscle and prevents airway irritation. It may be advantageous as it decreases the postoperative hoarseness and irritation due to tracheal tube. Further studies may be required to assess these airway-related complications and their relation to get suppressed effectively by varying the doses of dexmedetomidine.
Another important concern which our study highlighted was emergence as it is of utmost importance to conduct a neurological examination as early as possible postemergence. Our study revealed that dexmedetomidine infusion in a dose of 0.2 μg/kg/h did not delay emergence as compared to the placebo group though it did alleviate the stress and airway responses by effectively suppressing the cough and hemodynamic pertubations. However, as the infusion dose, was increased to 0.4 μg/kg/h; there was a delay in the emergence which was statistically, but not clinically significant. Further dose response studies may be required to determine the optimal infusion dose of dexmedetomidine in neurosurgical patients.
Shukry et al.[23] studied effects of infusions of dexmedetomidine (0.2 μg/kg/h) during surgery on the incidence of emergence delirium in 50 children aged 1–10 years scheduled for sevoflurane-based general anesthesia. Following inhalation induction, the children were randomly assigned to dexmedetomidine or placebo groups, respectively. The infusion of 0.2 μg/kg/h dexmedetomidine or equal volume of saline was started after securing the airway. The authors found that the incidence of emergence agitation decreased from 61% to 26%. In this study, the time to extubation and eye-opening were not different with dexmedetomidine versus placebo which was similar to our study.
Many studies have shown that dexmedetomidine, such as all other alpha-2 adrenoceptor agonists, has very little effect on ventilation, and there is no difference in respiratory function in patients who received dexmedetomidine compared to ones who received placebo following extubation.[252627] Our study also did not reveal respiratory complications or any difference in ventilation and respiratory function in the three study groups following extubation.
Limitation of the study
The only limitation of our study was that the patient population was not uniform as to various neurological ailments. The patients who underwent epilepsy surgery were also included in the study population who were on multiple antiepileptic drugs. These antiepileptic drugs are known to cause sedation and prolong the emergence time which could have altered the results of our study. However, in our study, none of the patients who underwent epilepsy surgery had a delayed emergence.
CONCLUSION
Our study in 90 patients undergoing craniotomy for various neurosurgical indications, demonstrates that dexmedetomidine infusion in doses of 0.2 and 0.4 μg/kg/h, effectively suppresses cough and attenuates hemodynamic responses to tracheal extubation without delaying emergence when compared to placebo.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Attenuation of cardiovascular responses to tracheal extubation with diltiazem. Anesth Analg. 1995;80:1217-22.
- [Google Scholar]
- Combined diltiazem and lidocaine reduces cardiovascular responses to tracheal extubation and anesthesia emergence in hypertensive patients. Can J Anaesth. 1999;46:952-6.
- [Google Scholar]
- Attenuation of cardiovascular responses to tracheal extubation: Verapamil versus diltiazem. Anesth Analg. 1996;82:1205-10.
- [Google Scholar]
- A prospective, comparative trial of three anesthetics for elective supratentorial craniotomy. Propofol/fentanyl, isoflurane/nitrous oxide, and fentanyl/nitrous oxide. Anesthesiology. 1993;78:1005-20.
- [Google Scholar]
- Labetalol and esmolol in the control of hypertension after intracranial surgery. Anesth Analg. 1990;70:68-71.
- [Google Scholar]
- Minimum alveolar concentration of desflurane for tracheal extubation in deeply anaesthetized, unpremedicated children. Br J Anaesth. 1997;78:370-1.
- [Google Scholar]
- Prostaglandin E1 attenuates the hypertensive response to tracheal extubation. Can J Anaesth. 1996;43:678-83.
- [Google Scholar]
- Blood-pressure and pulse-rate responses to endotracheal extubation with and without prior injection of lidocaine. Anesthesiology. 1979;51:171-3.
- [Google Scholar]
- Lidocaine sprayed down the endotracheal tube attenuates the airway-circulatory reflexes by local anesthesia during emergence and extubation. Anesth Analg. 2003;96:293-7.
- [Google Scholar]
- The efficacy of esmolol to blunt the haemodynamic response to endotracheal extubation in lumbar disc surgery. Res J Med Sci. 2008;2:99-104.
- [Google Scholar]
- Prophylactic esmolol infusion for the control of cardiovascular responses to extubation after intracranial surgery. Ann Acad Med Singapore. 2000;29:447-51.
- [Google Scholar]
- Comparison of nicardipine versus esmolol in attenuating the hemodynamic responses to anesthesia emergence and extubation. J Cardiothorac Vasc Anesth. 2007;21:45-50.
- [Google Scholar]
- Fentanyl attenuates cardiovascular responses to tracheal extubation. Acta Anaesthesiol Scand. 1995;39:85-9.
- [Google Scholar]
- Haemodynamic responses to extubation after cardiac surgery with and without continued sedation. Br J Anaesth. 1998;80:834-6.
- [Google Scholar]
- Hemodynamic responses to endotracheal extubation after coronary artery bypass grafting. Anesth Analg. 1991;73:10-5.
- [Google Scholar]
- Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816-20.
- [Google Scholar]
- Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability. Anaesthesia. 1997;52:736-44.
- [Google Scholar]
- Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49:1088-91.
- [Google Scholar]
- Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134-42.
- [Google Scholar]
- Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth. 2005;15:1098-104.
- [Google Scholar]
- Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658-65.
- [Google Scholar]
- Dexmedetomidine and neurocognitive testing in awake craniotomy. J Neurosurg Anesthesiol. 2004;16:20-5.
- [Google Scholar]
- Differential effects of lidocaine and remifentanil on response to the tracheal tube during emergence from general anaesthesia. Br J Anaesth. 2011;106:410-5.
- [Google Scholar]
- A simple study of awareness and dreaming during anaesthesia. Br J Anaesth. 1970;42:535-42.
- [Google Scholar]






