Translate this page into:
Alcohol-induced headaches: Evidence for a central mechanism?
Address for correspondence: Dr. Alessandro Panconesi, Via Amedeo Bassi 20, 50025 Montespertoli, FI, Italy. E-mail: a.panconesi@usl11.toscana.it
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Alcoholic drinks (ADs) have been reported as a migraine trigger in about one-third of the migraine patients in retrospective studies. Some studies found that ADs trigger also other primary headaches. The studies concerning the role of ADs in triggering various types of primary headaches published after the International Headache Society classification criteria of 1988 were reviewed, and the pathophysiological mechanisms were discussed. Many studies show that ADs are a trigger of migraine without aura (MO), migraine with aura (MA), cluster headache (CH), and tension-type headache (TH). While data on MO and CH are well delineated, those in MA and TH are discordant. There are sparse reports that ADs are also triggers of less frequent types of primary headache such as familial hemiplegic migraine, hemicrania continua, and paroxysmal hemicrania. However, in some countries, the occurrence of alcohol as headache trigger is negligible, perhaps determined by alcohol habits. The frequency estimates vary widely based on the study approach and population. In fact, prospective studies report a limited importance of ADs as migraine trigger. If ADs are capable of triggering practically all primary headaches, they should act at a common pathogenetic level. The mechanisms of alcohol-provoking headache were discussed in relationship to the principal pathogenetic theories of primary headaches. The conclusion was that vasodilatation is hardly compatible with ADs trigger activity of all primary headaches and a common pathogenetic mechanism at cortical, or more likely at subcortical/brainstem, level is more plausible.
Keywords
Alcohol
headache
migraine
migraine pathogenesis
trigger
Introduction
Many foods are considered capable of triggering migraine attack, but the relationship is frequently equivocal.[1] Perhaps, only alcohol has what is to be considered a sure dietary trigger, but its importance is still debated. Many retrospective studies show that alcoholic drinks (ADs) act as migraine triggers, at least occasionally, in about one-third of migraine patients, and as frequent/consistent trigger in about 10% of patients.[2] Some studies report that ADs are also a trigger of tension-type headache (TH).[1]
In the International Headache Society (IHS) classification, alcohol-induced headache is included as secondary headaches, in the section “Headache attributed to a substance or its withdrawal.”[3] However, problems for the classification of headache triggered by alcohol using IHS criteria were recently discussed.[4] One is the differentiation between hangover headache and migraine attack triggered by alcohol in diagnosed migraine patients.[567]
Alcohol being a common trigger in the principal types of primary headaches, suggest that these headaches can share a pathogenetic mechanism and that this trigger acts at the start of the neuronal pathway involved in headache provocation. To define this important issue, we have reviewed alcohol as a trigger of primary headaches and discussed the possible correlation of the results with the principal pathogenetic theories of the primary headaches.
Methods
This review was performed using a literature search on PubMed from 1988 (date of the first IHS classification) to December 2014. Search terms of “alcohol,” “wine,” “food trigger,” “dietary trigger,” “migraine,” “headache” were used. Additional sources were identified via manual search of bibliographies, references lists, and previous peer reviews. Review was restricted to studies written in English. Original studies were selected if they reported in the results a numeric percentage of headache patients referring any ADs as a trigger factor. Thirty-five papers were found corresponding to these criteria. Other studies useful for the correlation of the results with the pathogenesis of the primary headaches where also selected.
Results
Migraine without aura
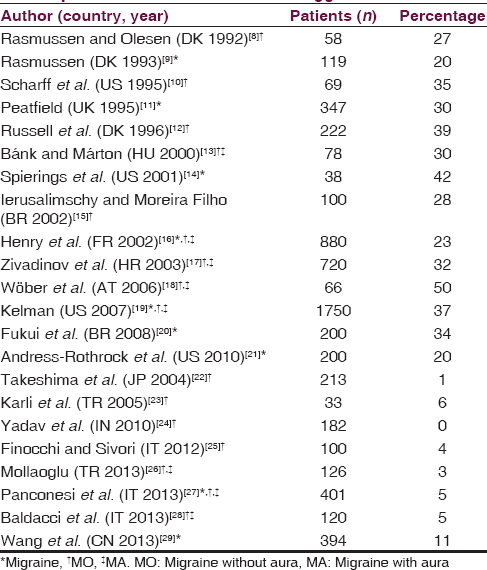
A recent review reports that in retrospective studies performed in different countries, about one-third of migraine patients indicate alcohol as a migraine trigger, and all ADs may act as trigger.[2] Fourteen studies reveal a percentage higher than 20% (mean 31.9%) [Table 1].[89101112131415161718192021] However, some of these studies show that alcohol acts as a trigger at least occasionally in a high percentage, but as a frequent/consistent trigger in only 10% of patients.[1819] In other eight studies performed in India, Japan, Turkey, China, and Italy, the percentages of alcohol or wine as migraine without aura (MO) trigger are very low (0–11%).[2223242526272829] In Italy and Turkey, the low percentage was confirmed by many studies.[2325262728] Prospective studies on alcohol as migraine trigger are few. One prospective study carried out in Austria (the Pamina study), examines a wide spectrum of factors related to migraine through the application of sophisticated statistical analysis, and provides evidence for the limited importance of nutrition (comprising alcoholic beverages) in the precipitation of migraine.[30] A broad observational general practice study (Spain, France, Italy) reports alcohol as a trigger in 12% of migraine patients but prospectively only in 4% of migraine attacks.[31]
Migraine with aura
Six studies report ADs as a trigger of migraine with aura (MA), four of them by the same Danish group [Table 2].[81217193233] Four studies report ADs trigger MA attack in a percentage similar to that found in MO patients (about 30%). In these studies, MA coexists with MO in a variable percentage of patients or this was not specified.[12171933] Two studies report a much lesser percentage (<10%).[832] Other studies, carried out in Japan, Turkey, and Italy, find that ADs never precipitated MA, but these studies also report that ADs rarely precipitate MO and TH.[222327]
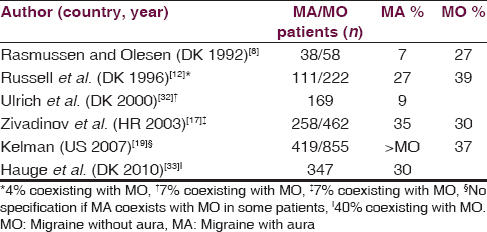
A recent detailed study shows a high number of factors that triggered MA attack, and more importantly, a high number of factors that frequently precipitated MA attack.[33] In this study, about 30% of patients with current MA (at least one attack within the last year) report ADs as a trigger, and only about 10% indicate ADs to trigger often or always an attack. For patients having both current MA and MO attacks, ADs are reported as a trigger of MO in 51% of patients and of MA in 40% of MA patients. In a further evaluation of patients indicating at least one trigger factor where exposure often or always triggers an attack of MA, 17% of them report ADs as a trigger: In 90% of these cases, ADs cause only 0–25% of their MA attacks while in the other 10% of cases, 26–50% of attacks.[34] All ADs trigger MA attacks, in 80% of cases within 3 h, and consistent with other studies, red wine is frequently indicated.[1217] A prospective study shows that white wine, red wine, beer, and spirits do not influence the risk of MA, similarly to MO or headache, while sparkling wine increases the risk of MA.[35]
Tension-type headache
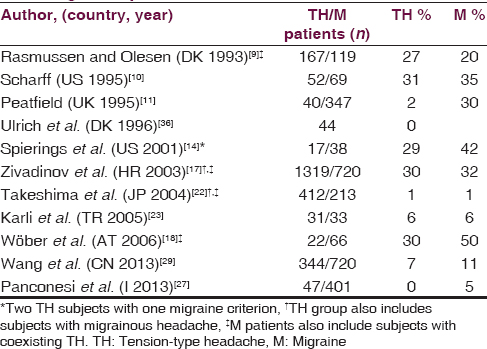
Five studies report ADs as TH trigger in approximately the same percentage (30%) of MO patients [Table 3].[910141718] However, the most extensive study also includes TH patients with coexisting migrainous headaches.[17] Peatfield find that 21% of TH with migraine features, but only 2% of pure TH, report sensitivity to ADs while Ulrich states that ADs provoke TH in MA and MO patients but not in TH patients.[1136] In these two studies, red wine is the most common ADs involved in triggering TH attacks, in accordance with another study.[18] Other studies find that ADs rarely precipitated both migraine and TH.[22232729] Furthermore, an old prospective study reports that ADs trigger vascular headaches but not TH.[37]
Cluster headache
More than 50% of patients indicate ADs are triggers of cluster headache (CH) attacks in chronic CH and in episodic CH only during bouts [Table 4].[3839404142434445] Studies in Asiatic population, similarly to an older study on Italian population, show a smaller percentage of CH patients reporting ADs as a trigger, which may be due in part to different alcohol habits.[46474849] This great variability appears even considering only CH patients who consume alcohol.[394647]
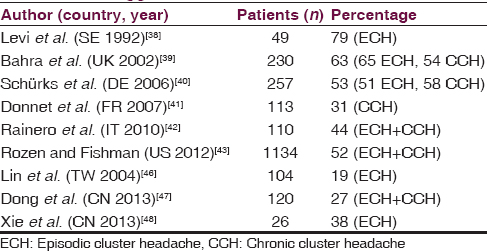
One study shows that ADs trigger CH attack within 2 h in 82% of cases, red wine being the most implicated.[40] However, others report beer as the most common trigger.[43] Curiously, several reports show transitory remission as a result of alcohol consumption and also delay of the following attack through consumption of large amounts of alcohol.[4045] Almost 50% of CH patients stated they drank alcohol.[40414347] Some reports suggest increased alcohol consumption, and even alcohol abuse, in CH population.[5051] Other studies do not support this, finding almost 50% change drinking habits, avoiding alcohol.[5253] In fact, CH patients reduce alcohol consumption during cluster period.[384351]
Other primary headaches
A recent Danish study reports that familial hemiplegic migraine (FHM) share environmental migraine triggers with MA and MO, including ADs in 15% of patients.[54] Hemicrania continua is exacerbated by alcohol within 3 h (38% of patients).[55] Of the other trigeminal autonomic cephalalgias (TACs), paroxysmal hemicrania is reported to be triggered by alcohol while there are no reports for short-lasting unilateral neuralgiform headache.[56]
Discussion
Many of retrospective studies report that ADs trigger migraine attack in about one-third of MO population. A higher percentage of patients (over 50%) referring ADs as a trigger is found in CH. The role of alcohol in other types of primary headaches is less uniformly defined. The more detailed study shows that ADs are a trigger of MA attack in a similar percentage to MO attack,[33] confirming the data of earlier studies,[121719] but not the low percentage found in previous studies of the same Danish group.[832] The same Danish group reports that the typical triggers of FHM are the same of MA, including ADs.[54] The role of alcohol in TH is less surely defined. While many studies show that ADs trigger headache in TH patients in approximately the same percentage (30%) as in migraine patients, other studies show that ADs trigger TH in migraine patients[36] but not in patients with pure TH.[1127] Moreover, the larger study also includes TH patients with coexisting migrainous headaches.[17] This wide variability may results from the similar phenotypic features between MO and TH while MA and CH have more distinctive characteristics.[3] ADs have been reported to trigger even more rare forms of primary headaches such as FHM, hemicrania continua, and paroxysmal hemicranias.[545556]
The interval between ADs intake and the start of MO is not well determined in many studies. However, it is reported that in 80% of cases, ADs can trigger MA attacks and CH attacks within 3 and 2 h, respectively; consistent with other studies,[1217] red wine is the principal alcohol trigger.[3440] Even MO induced experimentally by red wine developed for the most part within 3 h.[57] However, all ADs provoke headache and the type of beverage most frequently consumed in a country will probably be the type of ADs most commonly reported to trigger headache.[243]
In contrast to the well-defined role of ADs in MO patients found in many studies, in India, Japan, Turkey, China, and Italy, a much lower percentages of MO patients indicate ADs as a trigger, perhaps partly due to alcohol habits, that is to lower consumption or different beverage strength to Europe and US.[2223242526272829] Cultural differences can be responsible even of the very low percentage of MA and TH patients and of the lower percentage of CH patients referring ADs as a trigger found in these countries.[22232729464748] In fact, in comparison to Europe and the US, the percentage of abstainers in India and Turkey is much higher and the alcohol consumption per capita is much lower, but this assumption is not valid for Italy and Japan. Conversely, in Brazil, the percentage of abstainers is high while the percentage of MO patients sensitive to ADs is equal to that found in Europe and US.[58] In addition to the population, the frequency estimates vary widely based on the study approach. Reported rates of trigger factors vary with the method of the study (retrospective vs. prospective, spontaneous report vs. checklists, population vs. clinic based), and many methodological difficulties of investigation was highlighted.[59606162] Differently to retrospective studies, influenced by recall bias, few prospective studies provide evidence for the absence or very limited role of ADs in the precipitation of migraine.[303135]
Many population-based studies, carried in various countries show an inverse relationship between alcohol and migraine, both in MO than MA, and nonmigraine headache.[463] Recently, two studies show that patients with chronic migraine compared to patients with chronic TH and with patients without headache, less likely drink alcohol.[2764] A possible explanation for the inverse association between alcohol use and headache disorders is that subjects with headache disorders may be abstaining from alcohol as it is a trigger for their headache attack. In fact, 90% of MA patients sensitive to ADs report abstaining or avoiding certain type of alcohol and CH patients reduce alcohol consumption during cluster periods.[34384351] However, some observations seem not to support the explication that migraine patients consume less ADs because they trigger migraine attacks: (1) Among migraine patients who did not drink alcohol at all (about 50%), only 3% reported that abstaining from alcohol was a result of alcohol as a migraine trigger;[27] (2) differently with we can expect if ADs trigger migraine, an higher use of ADs was reported in chronic migraine in comparison whit episodic migraine;[64] (3) the percentage of subjects who never or seldom consume ADs is higher in migraine and nonmigraine headache,[636566] which suggests that factors other than previous experience of alcohol as a trigger can contribute to reduced alcohol consumption in migraine, such as personal preference.[4]
The possible triggering site
However, if ADs are a trigger factor of virtually all primary headaches, a fundamental question emerges: That is if ADs act at different levels in triggering the primary headaches or do they act at an initial level of a common pathogenetic pathway. The second hypothesis is more plausible, because it seems unlikely that ADs may act with different mechanisms in different forms of headaches but with similar phenotypic features.
Meningeal nociceptors activation through inflammatory/vasodilatatory mechanism is suggested responsible of migraine pain: However, how these nociceptors are activated remains largely speculative. Cortical spreading depression (CSD), a transient neuronal and glial depolarization that propagates slowly across the cerebral cortex, is the putative electrophysiological event underlying migraine aura and has been proposed as the mechanism responsible for the activation of the migraine pain pathway, but many arguments against its role are reported.[67] Whether the pain in TH originates from myofascial tissues or from central mechanisms in the brain is still a matter for debate. However, the present consensus is that peripheral pain mechanisms have a role in episodic TH whereas central dysnociception is predominant in chronic TH.[68] Several studies support the hypothalamic involvement in the TACs pathophysiology.[69]
Concerning migraine pain, animal studies report that alcohol, mimicking capsaicin, provokes neurogenic inflammation in the trigeminovascular system, and vasodilatation of meningeal vessels through calcitonin gene-related peptide (CGRP) release from perivascular sensory nerve terminals.[70] Many studies with noninvasive imaging techniques has well established that low-moderate doses of alcohol, after oral or intravenous (alcohol clamp) administration, increases cerebral blood flow. The increased cerebral perfusion, due to direct or indirect vasodilatory mechanisms, was found in most cerebral regions, stronger in women, and inversely correlated with sensitivity of alcohol.[717273] Therefore, alcohol may have an action similar to other strong vasodilators such histamine, CGRP, and glyceryl trinitrate (GTN), which trigger migraine. However, disagreement between cranial vasodilatation and drug-provoked headache suggests that vasodilatation per se could not explain the induced headache.[747576]
If the vasodilator action of alcohol at the trigeminovascular level can theoretically be compatible with MO and CH provocation, how can it be the trigger of aura and subsequent migraine pain or TH pain?
Other vasodilating drugs such as CGRP and nitroglycerin/GTN failed to induce migraine-like attacks and aura in patients with FHM, while in a small percentage of patient with MA, they have been reported to provoke aura symptoms associated with migraine-like headache, but a role of experimental stress cannot be excluded.[77787980] On the other hand, there is no description in publications that histamine triggers MA. It is also difficult to sustain a direct vasodilating action of alcohol in the triggering TH if we do not believe in a pathogenethic mechanism in common between migraine and TH.
Migraine triggers, included ADs, can theoretically provoke CSD which can theoretically be responsible for MA, but also for FHM and MO. But at what level do ADs trigger CH, other TACs and TH? However, ethanol infusion decreases the propagation rate of CSD, indicating a decline of tissue excitability and in the CSD initiation mechanisms.[81] Moreover, acute intake of ethanol acts as a central nervous system depressant and at cortical level, alcohol is reported to reduce cortical excitability or facilitate the activity of cortical inhibitory circuits probably through the increase of gamma-aminobutyric acid neurotransmission.[8283]
Therefore, if ADs are definitely confirmed a common trigger of various primary headaches, some of which with phenotypic overlap, it is more plausible that they act probably at a common central cortical or subcortical levels.
A unitary hypothesis suggests that migraine triggers promote headache by the activation of subcortical distinct neuronal pathways convergent into parasympathetic innervation, which if activated results in meningeal release of vasoactive and algesic mediators capable of activating meningeal nociceptors.[84] Another view sustains that migraine headache can be triggered in absence of nociceptor activation, simply as a consequence of disinhibition of tonic control from antinociceptive system, which may result in abnormal central processing of normal sensory signal,[8586] a mechanism previously theorized as “functional deafferentation.”[87]
Another important question, previously discussed, is whether alcohol per se or some components of AD are responsible of headache provocation.[24] However, the analgesic activity of alcohol deserve to be briefly discussed because not easily compatible with headache triggered by ADs. In fact, the anesthetic and analgesic properties of alcohol have been recorded for centuries and alcohol is frequently used as self-medication in pain syndromes.[8889] Some experiments show that alcohol have analgesic effect in the early hours after their administration, which is the amount of time ADs have been reported to trigger MO, MA, and CH. In fact, rats that received dural stimulation followed by alcohol showed an initial analgesic effect within the first 2 h after alcohol ingestion; however, 4–6 h later, their pain sensitivity increased.[90] Similarly, intravenously administered alcohol has an analgesic effect in humans,[91] while hyperalgesia is found in alcohol withdrawal.[92] Interestingly, many experiments with “alcohol clamp,” a method of infusing alcohol to achieve and maintain a target breath/blood alcohol level for a prolonged time (3 h), do not report migraine within the 8 h of the typical study session in several hundred subjects.[4919394] Only one study carried out on few subjects reports mild and transient headache as side effect in 17–25% of Japanese but not Caucasians male subjects.[95] Experiments with this technique in migraine patients should be of much interest.
Conclusion
ADs have been reported to trigger the principal types of primary headaches. Certainly, ADs, even in small doses, trigger headache in some MO patients, but what is debated is the degree, which depend, in part, from the population studied, the country where the study was performed and the study approach. While the results in MO and CH are in relative agreement, those in MA and TH are discordant. However, if the role of ADs in triggering MA and TH will be confirmed, a common trigger site should be considered. In this case, a direct action at the vascular system is hardly compatible with TH or MA. More plausible is an action at subcortical pain modulatory circuits, which in some way stimulate the neural generator of CH (hypothalamus?) and of migraine aura (cortex?, thalamus?).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Alcohol and migraine: Trigger factor, consumption, mechanisms. A review. J Headache Pain. 2008;9:19-27.
- [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629-808.
- [Google Scholar]
- Alcohol and migraine: What should we tell patients? Curr Pain Headache Rep. 2011;15:177-84.
- [Google Scholar]
- Hangover headache: Accompanying symptoms. VAGA study of headache epidemiology. J Headache Pain. 2004;5:224-9.
- [Google Scholar]
- Frequency and features of delayed alcohol-induced headache among university students. Headache. 2006;46:688-91.
- [Google Scholar]
- Alcohol consumption and hangover patterns among migraine sufferers. J Neurosci Rural Pract. 2014;5:128-34.
- [Google Scholar]
- Migraine with aura and migraine without aura: An epidemiological study. Cephalalgia. 1992;12:221-8.
- [Google Scholar]
- Migraine and tension-type headache in a general population: Precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53:65-72.
- [Google Scholar]
- Triggers of headache episodes and coping responses of headache diagnostic groups. Headache. 1995;35:397-403.
- [Google Scholar]
- Relationships between food, wine, and beer-precipitated migrainous headaches. Headache. 1995;35:355-7.
- [Google Scholar]
- Migraine without aura and migraine with aura are distinct clinical entities: A study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996;16:239-45.
- [Google Scholar]
- Precipitating and aggravating factors of migraine versus tension-type headache. Headache. 2001;41:554-8.
- [Google Scholar]
- Precipitating factors of migraine attacks in patients with migraine without aura. Arq Neuropsiquiatr. 2002;60:609-13.
- [Google Scholar]
- Prevalence and clinical characteristics of migraine in France. Neurology. 2002;59:232-7.
- [Google Scholar]
- Migraine and tension-type headache in Croatia: A population-based survey of precipitating factors. Cephalalgia. 2003;23:336-43.
- [Google Scholar]
- Trigger factors of migraine and tension-type headache: Experience and knowledge of the patients. J Headache Pain. 2006;7:188-95.
- [Google Scholar]
- The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394-402.
- [Google Scholar]
- An analysis of migraine triggers in a clinic-based population. Headache. 2010;50:1366-70.
- [Google Scholar]
- Population-based door-to-door survey of migraine in Japan: The Daisen study. Headache. 2004;44:8-19.
- [Google Scholar]
- Comparison of pre-headache phases and trigger factors of migraine and episodic tension-type headache: Do they share similar clinical pathophysiology? Cephalalgia. 2005;25:444-51.
- [Google Scholar]
- Food as trigger and aggravating factor of migraine. Neurol Sci. 2012;33(Suppl 1):S77-80.
- [Google Scholar]
- Triggers of migraine and tension-type headache in China: A clinic-based survey. Eur J Neurol. 2013;20:689-96.
- [Google Scholar]
- Prospective analysis of factors related to migraine attacks: The PAMINA study. Cephalalgia. 2007;27:304-14.
- [Google Scholar]
- Influence of trigger factors on the efficacy of almotriptan as early intervention for the treatment of acute migraine in a primary care setting: The START study. Expert Rev Neurother. 2010;10:1399-408.
- [Google Scholar]
- Possible risk factors and precipitants for migraine with aura in discordant twin-pairs: A population-based study. Cephalalgia. 2000;20:821-5.
- [Google Scholar]
- Characterization of consistent triggers of migraine with aura. Cephalalgia. 2011;31:416-38.
- [Google Scholar]
- Prospective analysis of factors related to migraine aura – The PAMINA study. Headache. 2012;52:1236-45.
- [Google Scholar]
- A comparison of tension-type headache in migraineurs and in non-migraineurs: A population-based study. Pain. 1996;67:501-6.
- [Google Scholar]
- Dietary precipitation of vascular headaches. In: Chandra RK, ed. Food Allergy. St. John's, Newfoundland: Nutrition Research Education Foundation; 1987. p. :237-52.
- [Google Scholar]
- Episodic cluster headache. II: High tobacco and alcohol consumption in males. Headache. 1992;32:184-7.
- [Google Scholar]
- Cluster headache: A prospective clinical study with diagnostic implications. Neurology. 2002;58:354-61.
- [Google Scholar]
- Cluster headache: Clinical presentation, lifestyle features, and medical treatment. Headache. 2006;46:1246-54.
- [Google Scholar]
- Chronic cluster headache: A French clinical descriptive study. J Neurol Neurosurg Psychiatry. 2007;78:1354-8.
- [Google Scholar]
- Cluster headache is associated with the alcohol dehydrogenase 4 (ADH4) gene. Headache. 2010;50:92-8.
- [Google Scholar]
- Cluster headache in the United States of America: Demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52:99-113.
- [Google Scholar]
- Cluster headache in the Taiwanese – A clinic-based study. Cephalalgia. 2004;24:631-8.
- [Google Scholar]
- Clinical profile of cluster headaches in China – A clinic-based study. J Headache Pain. 2013;14:27.
- [Google Scholar]
- Clinical features of cluster headache: An outpatient clinic study from China. Pain Med. 2013;14:802-7.
- [Google Scholar]
- Cluster headache and lifestyle: Remarks on a population of 374 male patients. Cephalalgia. 1999;19:88-94.
- [Google Scholar]
- Predictors of hazardous alcohol consumption among patients with cluster headache. Cephalalgia. 2006;26:623-7.
- [Google Scholar]
- Familial cluster headache: Demographic patterns in affected and nonaffected. Headache. 2010;50:374-82.
- [Google Scholar]
- Hemicrania continua: A clinical study of 39 patients with diagnostic implications. Brain. 2010;133(Pt 7):1973-86.
- [Google Scholar]
- Paroxysmal hemicrania: A prospective clinical study of 31 cases. Brain. 2008;131(Pt 4):1142-55.
- [Google Scholar]
- Global Status Report on Alcohol 2004. 2004. World Health Organization. Available from: http://www.faslink.org/WHO_global_alcohol_status_report_2004.pdf
- [Google Scholar]
- Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache. 2014;54:1670-9.
- [Google Scholar]
- Migraine and triggers: Post hoc ergo propter hoc? Curr Pain Headache Rep. 2013;17:370.
- [Google Scholar]
- Natural experimentation is a challenging method for identifying headache triggers. Headache. 2013;53:636-43.
- [Google Scholar]
- Reliability of assessing lifestyle and trigger factors in patients with migraine – Findings from the PAMINA study. Eur J Neurol. 2016;23:120-6.
- [Google Scholar]
- Association between migraine, lifestyle and socioeconomic factors: A population-based cross-sectional study. J Headache Pain. 2011;12:157-72.
- [Google Scholar]
- Epidemiological profiles of patients with chronic migraine and chronic tension-type headache. J Headache Pain. 2013;14:40.
- [Google Scholar]
- Migraine frequency and risk of cardiovascular disease in women. Neurology. 2009;73:581-8.
- [Google Scholar]
- Headache is associated with lower alcohol consumption among medical students. Arq Neuropsiquiatr. 2011;69:620-3.
- [Google Scholar]
- A critical view on the role of migraine triggers in the genesis of migraine pain. Headache. 2009;49:953-7.
- [Google Scholar]
- Tension-type headache: Current research and clinical management. Lancet Neurol. 2008;7:70-83.
- [Google Scholar]
- The hypothalamus: Specific or nonspecific role in the pathophysiology of trigeminal autonomic cephalalgias? Curr Pain Headache Rep. 2011;15:101-7.
- [Google Scholar]
- Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the Guinea pig. Cephalalgia. 2008;28:9-17.
- [Google Scholar]
- Alcohol-induced changes in cerebral blood flow and cerebral blood volume in social drinkers. Alcohol Alcohol. 2013;48:160-5.
- [Google Scholar]
- Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. J Cereb Blood Flow Metab. 2014;34:472-9.
- [Google Scholar]
- Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology (Berl). 2015;232:733-44.
- [Google Scholar]
- Migraine pain: Reflections against vasodilatation. J Headache Pain. 2009;10:317-25.
- [Google Scholar]
- Lack of correlation between vasodilatation and pharmacologically induced immediate headache in healthy subjects. Cephalalgia. 2011;31:683-90.
- [Google Scholar]
- Discrepancy between strong cephalic arterial dilatation and mild headache caused by prostaglandin D2 (PGD2) Cephalalgia. 2011;31:65-76.
- [Google Scholar]
- Calcitonin gene-related peptide does not cause migraine attacks in patients with familial hemiplegic migraine. Headache. 2011;51:544-53.
- [Google Scholar]
- Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain. 2004;110:675-80.
- [Google Scholar]
- Reliability of the nitroglycerin provocative test in the diagnosis of neurovascular headaches. Cephalalgia. 2004;24:110-9.
- [Google Scholar]
- Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179-86.
- [Google Scholar]
- The effect of ethanol on metabolic, hemodynamic and electrical responses to cortical spreading depression. Brain Res. 2001;908:174-86.
- [Google Scholar]
- Alcohol reduces prefrontal cortical excitability in humans: A combined TMS and EEG study. Neuropsychopharmacology. 2003;28:747-54.
- [Google Scholar]
- Acute and chronic effects of ethanol on cortical excitability. Clin Neurophysiol. 2008;119:667-74.
- [Google Scholar]
- Unitary hypothesis for multiple triggers of the pain and strain of migraine. J Comp Neurol. 2005;493:9-14.
- [Google Scholar]
- Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007;13:39-44.
- [Google Scholar]
- Effect of cortical spreading depression on basal and evoked traffic in the trigeminovascular sensory system. Cephalalgia. 2011;31:1439-51.
- [Google Scholar]
- Quasi-phantom head pain from functional deafferentation. Clin J Pain. 1987;3:63-80.
- [Google Scholar]
- The effect of nonrecurring alcohol administration on pain perception in humans: A systematic review. J Pain Res. 2015;8:175-87.
- [Google Scholar]
- Interrelations between pain and alcohol: An integrative review. Clin Psychol Rev. 2015;37:57-71.
- [Google Scholar]
- Ethanol and pain sensitivity: Effects in healthy subjects using an acute pain paradigm. Alcohol Clin Exp Res. 2008;32:952-8.
- [Google Scholar]
- Intravenous ethanol infusion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239-46.
- [Google Scholar]
- Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467-77.
- [Google Scholar]
- A comparison of the central nervous system effects of alcohol at pseudo-steady state in Caucasian and expatriate Japanese healthy male volunteers. Alcohol. 2012;46:657-64.
- [Google Scholar]






