Translate this page into:
Alcohol consumption and hangover patterns among migraine sufferers
Address for correspondence: Dr. Yair Zlotnik, Department of Neurology, Soroka University Medical Center, POD 151, Beer-Sheva - 84101, Israel. E-mail: zlotniky@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims:
Alcohol hangover is a poorly understood cluster of symptoms occurring following a heavy consumption of alcohol. The term “delayed alcohol-induced headache” is often used synonymously. Our objective was to compare alcohol hangover symptoms in migraine sufferers and nonsufferers.
Materials and Methods:
In this cross-sectional study, university students were asked to fill structured questionnaires assessing headache history, alcoholic consumption, and hangover symptoms (using the Hangover Symptom Scale (HSS)). Subjects were classified as suffering from migraine with or without aura and nonsufferers according the International Classification of Headache Disorders 2nd Edition (ICHD-II). The 13 hangover symptoms were divided by the researches into migraine-like and other nonmigraine-like symptoms.
Results:
Hangover symptoms among 95 migraine sufferers and 597 nonsufferers were compared. Migraine sufferers consumed less alcohol compared with the nonsufferers (mean drinks/week 2.34 ± 4.11 vs. 2.92 ± 3.58, P = 0.038) and suffered from higher tendency to migraine-like symptoms after drinking (mean 2.91 ± 3.43 vs. 1.85 ± 2.35, P = 0.002) but not to other hangover symptoms (mean 5.39 ± 6.31 vs. 4.34 ± 4.56, P = 0.1).
Conclusions:
Migraine sufferers consume less alcohol, especially beer and liquors, and are more vulnerable to migraine-like hangover symptoms than nonsufferers. The finding that the tendency to develop migraine attacks affects the hangover symptomatology may suggest a similarity in pathophysiology, and possibly in treatment options.
Keywords
Alcohol
delayed alcohol-induced headache
hangover
migraine
students
Introduction
Alcohol hangover, or “veisalgia”, is a well-known and fairly common phenomenon that generally occurs after heavy consumption of alcohol. It comprises a constellation of physical, cognitive, and psychological disturbances. Physical symptoms generally include headache (usually throbbing in nature), dizziness, anorexia, diarrhea, tremulousness, fatigue, and nausea. Tachycardia and sweating may also occur. Main cognitive and mood symptoms include decreased attention and concentration, decreased visuospatial skills, depression, anxiety, and irritability.[1]
Migraine is a primary episodic headache disorder characterized by various combinations of neurological, gastrointestinal, and autonomic changes. It is a common disorder (in the USA, 18% of women and 6% of men had at least one migraine attack in the previous year).[2] Before puberty, migraine prevalence is about 4%. After puberty, prevalence increases more rapidly in girls than in boys. Prevalence increases until about age 40 years, then declines.[3]
The typical headache attacks experienced by migraine sufferers is unilateral, of gradual onset, throbbing (85%),[4] moderate to marked in severity, and aggravated by movement.[5] Pain can be bilateral (40%) or start on one side and become generalized. It lasts 4-72 h in adults and 1-72 h in children.
Anorexia is common. Nausea occurs in almost 90% of patients, while vomiting occurs in about a third.[6] Sensory hypersensitivity results in patients seeking a dark, quiet room. Blurry vision, nasal stuffiness, anorexia, hunger, tenesmus, diarrhea, abdominal cramps, polyuria, facial pallor, sensations of heat or cold, and sweating might occur. Depression, fatigue, anxiety, nervousness, irritability, and impairment of concentration are common. After the headache, the patient often feels tired, washed out, irritable, or listless, and can have impaired concentration, scalp tenderness, or mood changes. Some feel unusually refreshed or euphoric after an attack; others experience depression and malaise.[7]
Many studies in different countries show that alcohol is a headache trigger in a high percentage of migraine sufferers.[8] In the classification of the International Headache Society (IHS), two types of alcohol-induced headache were reported in secondary headaches (code 8.1.4): The immediate alcohol-induced headache, which develops within 3 h after ingestion of alcoholic beverages, and the delayed alcohol-induced headache (previously termed hangover headache), which develops after the blood alcohol level declines or reduces to zero. In the comment, it was stated that few subjects develop the former type of headache, while the alcohol-delayed headache is one of the commonest type of headache, the day after alcohol consumption, provoked by ingestion of modest amount of alcoholic beverages in migraine sufferers, while nonmigraine sufferers usually need a higher intake. The same classification stated that migraine could be aggravated (long-term increase) by frequent intake of alcoholic beverages. Nevertheless, the susceptibility to hangover symptoms of migraine patients compared with nonmigraine sufferers has not been determined.
The similarity between the symptomatology of these two common disorders, migraine and hangover, has led us to compare alcohol hangover symptoms in migraine sufferers and nonsufferers. We hypothesized that migraine sufferers are more susceptible to specific aspects of the hangover symptomatology complex, namely those that are similar to the migraine attack.
Materials and Methods
Study population
University students in Israel, aged 18-40 years, were randomly assigned to this cross-sectional study during September-October 2009. The study was approved by the Internal Review Board of our University Medical Center. Exclusion criteria from the study were: Self-reported head trauma/head or face surgery in the past year and self-reported chronic headache lasting more than 15 days per month.
Study instruments and variables
The participants were requested to fill questionnaires. The questionnaires were filled without the presence of an interviewer, and did not include any recognizable data to assure full anonymity of the collected data. The questionnaires included demographics, headache history and characteristics, health-related habits (smoking and alcohol consumption), and hangover symptoms.
Hangover symptoms
The presence and strength of hangover symptoms were accessed using the Hangover Symptom Scale (HSS). The scale was developed and validated by Slutske et al.[9] and was translated and validated to Hebrew by the researchers. The assessment of hangover symptoms contained 13 items that sampled from each of the eight domains (constitutional, pain, gastrointestinal, sleep and biological rhythms, sensory, cognitive, mood, and sympathetic hyperactivity symptoms) described by Swift and Davidson.[10] These 13 hangover symptoms were assessed with reference to the first few times the participants ever drank alcohol, and then repeated with reference to drinking occasions that occurred in the past 12 months. For each of the 13 hangover symptoms, the participants indicated the percentage of drinking occasions, on a 5-point scale ranging from never (0% of the time) to every time (100% of the time), that were followed the next morning by the symptom (one of the symptoms, vomiting, could have occurred either during the night or the next morning). Assessing the percentage of drinking occasions after which hangover symptoms occur partially controls for differences in the frequency of drinking and allows the HSS item scores to be interpreted as hangover susceptibility or proneness.
For the study purposes, the scale of 13 symptoms was split to two sub-scales. The sub-scale of migraine-like symptoms included the four following items: Headache, nausea, vomiting, and sensitivity to light and sound. The sub-scale of other symptoms included the nine following items: Woke up tired, difficulties falling asleep, thirst, tremor/chills, difficulties concentrating, anxious, weakness, filling depressed, and diaphoresis. The grades of the sub-scales and the whole HSS were calculated for each participant as sums of their items ranks (“polytomous approach”[9]) and used as the primary outcomes of the study. According the authors’ report, the internal reliability (Cronbach's alpha) for the scale was 0.86.[9] In our study population, the Cronbach's alpha values of HSS over the last year were 0.885, 0.752, and 0.831 for the total scale, migraine-like symptoms, and other symptoms, respectively.
Headache history
The participants were asked to report whether they suffered from troubling headache during the last 3 months and if so, to report headache characteristics and accompanying features, using a list of items (frequency, number of headache days/month, severity, aura symptoms, localization, quality, exacerbating factors, nausea, vomiting, sensitivity to light and sound, triggers, etc.). The participants’ responses to the questionnaires were reviewed by a neurologist and migraine diagnosis was assigned according to the International Classification of Headache Disorders 2nd Edition (ICHD-2) criteria for migraine with and without aura.[5]
The presence of migraine was defined as the “exposure” variable in the study. Participants were divided into two groups: Migraine (both with- and without aura) sufferers and nonmigraine sufferers. Among the migraine-sufferers, the data regarding the headache characteristics were collected using the Migraine Disability Assessment Questionnaire (MIDAS) – a five-item questionnaire developed to measure headache-related disability and improve doctor–patient communication about the functional consequences of migraine.[11] MIDAS is a brief and reliable headache-specific tool, which captures headache-related disability. Five questions investigate the influence of headache on everyday activities over the preceding 3 months. Items 1 and 2 investigate paid work, enquiring as to the number of days off work due to headache and the number of days where productivity was reduced by half or more. Items 3 and 4 ask the same questions about household work. Item 5 enquires about missed days of recreational, social, and family activities. The total score is obtained by summing the individual scores of the individual MIDAS items. From the score, one of four disability grades is assigned: Grade I, minimal or infrequent disability corresponds to a MIDAS score of 0-5; grade II, mild or infrequent disability corresponds to a MIDAS score of 6-10; grade III, moderate disability corresponds to a MIDAS score of 11–20; and grade IV, severe disability corresponds to a MIDAS score of ≥21. We hypothesized that higher MIDAS scores would be in correlation with lower amounts of alcohol consumption.
The demographic data, measure of alcoholic consumption, and smoking status of the participants were considered as the potential confounders.
Demographic data
Participants were asked to answer questions regarding their gender, age, familial status, country of their or parents’ origin.
Alcoholic consumption
Subjective report of the investigated subjects was used to assess alcohol consumption. Participants were asked to report the amount of alcohol measures (“drinks”) consumed in a typical drinking day (or night): 1/3 Liter of beer, glasses of wine and measures (“shots”) of spirit (liquor, vodka, arak, whiskey, etc.). The frequency of alcohol consumption was measured as the number of drinking days in a typical week. For each participant total measured alcohol consumption was calculated as amount of alcohol measures per week.
Smoking
Participants were asked about cigarette smoking and marijuana smoking habits. Students who reported marijuana smoking more than once were considered as marijuana smokers. Participants who answered affirmatively to the question “do you smoke cigarettes?” were considered as cigarette smokers.
Sample size justification
The sample size was calculated based on the following assumptions: 95% Confidence interval (2-sided), 80% power and the prevalence of migraine in the study sample of 15% (1:6). In addition, we assumed the mean difference of 1.5 points in sub-scale of migraine-like symptoms between the study groups with standard deviation of 3 points. Based on these assumptions, the calculated sample size was 259 participants (37 in the migraine-sufferers group and 222 in the nonsufferers group).
Statistical analysis
Data were analyzed using IBM® SPSS® Statistics, Version 20 software. The comparison of demographic characteristics, health-related habits, and each one of hangover symptoms between the groups of migraine sufferers and nonmigraine was performed using Chi-square test (for dichotomous and categorical data, e.g., sex, family status, alcohol consumption, cigarette smoking, and marihuana consumption); Student's t-test for continuous data (e.g., age and total HSS) and nonparametric Mann–Whitney U test for the variables of measured alcohol consumption and hangover symptoms by HSS scale.
Relationships between the investigated independent variables and the outcomes (migraine-like symptoms sub-scale, other symptoms sub-scale, and total HSS) in the univariate level were assessed using Student's t-test and Pearson correlation for the dichotomous and continuous independent variables, respectively. Since two sub-scales comprised the HSS, Bonferroni correction was performed and significance levels for these sub-scales were set at P = 0.025. Additionally, multivariate analysis of variance (MANOVA) was applied in which associations of the primary outcomes to each one of the investigated variables was assessed. Relationship between MIDAS score and alcoholic consumption was assessed using Spearman correlation.
Multivariate analysis by linear regression tested independent predictors for hangover symptoms (total HSS at last year and its sub-scales). For each test, P values less than 0.05 were considered as statistically significant unless stated otherwise.
Results
Study population and groups
A total of 739 questionnaires were handed to students; 47 of them met the exclusion criteria, therefore 692 questionnaires were studied. Ninety-five participants (14%) met the IHS criteria for episodic migraine with or without aura.
Participants in both migraine and nonmigraine groups were of similar averaged age (24.1, SD = 2.78 vs. 24.2, SD = 2.37; P = 0.604). In the migraine group, the percentage of women was significantly higher (83.2% vs. 47%; P > 0.001). There was no significant difference in the percentage of married participants between the migraine and nonmigraine groups (8.4% vs. 11.8%; P = 0.332).
MIDAS
Results of MIDAS score among migraine sufferers ranged from 0 to 70 with a mean of 12.52 and SD of 13.81, classified as medium grade disability.
Alcohol consumption
Most of the participants (81.07%) reported any alcohol consumption with no significant difference between the migraine and nonmigraine groups (78.95% vs. 81.41%; P = 0.570). Total measured alcoholic consumption among migraine sufferers was significantly lower than among nonmigraine sufferers. Of the specific alcoholic beverages that were studied, beer and spirits were found to be consumed significantly less among migraine sufferers. However, there was no difference in red wine consumption between the two groups [Table 1].
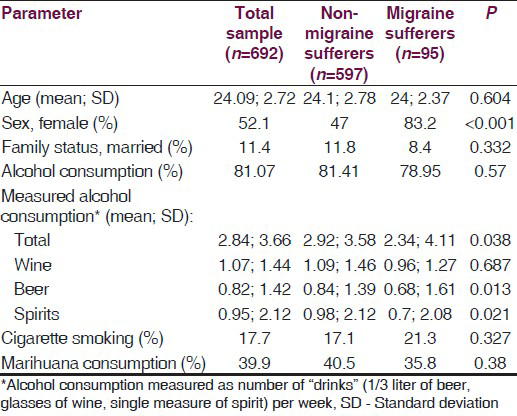
Among migraine sufferers, a weak significant negative correlation was found between the MIDAS score and total alcohol consumption (rs = −0.21, P = 0.049). Of the specific alcoholic beverages that were studied, the correlation coefficients (rs) were: −0.12 (P = 0.267), −0.24 (P = 0.026), and −0.02 (P = 0.881) for wine, beer, and spirits consumptions, respectively.
Hangover symptoms among migraine and non-migraine sufferers
Table 2 shows the mean (SD) values of hangover symptoms among “drinkers” (n = 539) and by groups of migraine and nonmigraine sufferers. Our data shows that the migraine sufferers tended to be more vulnerable to all hangover symptoms than nonmigraine sufferers. For the two out of four migraine-like symptoms (“headache on the morning after” and “nausea on the morning after”) the differences between the two groups were significant. In addition, significant difference between the two groups was found in only one out of nine other symptoms (“difficulties falling asleep”).

The sub-scale of migraine-like symptoms was highly correlated with the sub-scale of other symptoms (r = 0.773, P < 0.001).
Association between migraine and HSS scales
The relationships between the investigated variables and the primary outcomes are presented in Table 3. Migraine was associated with higher score of the migraine-like scale. However, no relationship was found between migraine and other hangover symptoms. Other variables, such as being single, spirits consumption, cigarette smoking, and marihuana consumption, were associated with higher scores on both sub-scales and total HSS as well.

The results of the multivariate analysis [Table 4a] show that migraine is associated with higher scores in the migraine-like symptom scale, but is not associated with other hangover symptoms after adjustment for the investigated variables.

Additionally we performed multivariable analysis separately by the gender groups [Table 4b and c]. The results of this analysis show that migraine is associated with higher scores in the migraine-like symptom scale in females only.
Discussion
While alcohol hangover is a common disorder,[10] causing suffering and disability to millions worldwide, there is no direct way to measure it. The HSS, which was used in this study, was developed to provide a useful hangover measure, assessing multiple symptom domains, which do not rely on respondents’ subjective definitions of hangover. Assessing the percentage of drinking occasions after which hangover symptoms allows the HSS item scores to be interpreted as hangover susceptibility or proneness.
The HSS also allows us to assess the hangover symptoms following early drinking experiences. The interpretation of this data should be cautious, since recall bias is a major concern. Nevertheless, Slutske et al.[9] reported that in college undergraduate students, the estimates of hangover for the first few drinking occasions and the past year were highly stable, what might be interpreted as indicating that hangover proneness is trait like. It is also possible that retrospective recall biases cause higher correlation between HSS scores across time periods.
Since migraine is a prevalent disorder, we sampled our study population from the general population. Participants were divided into two groups, migraine sufferers and nonmigraine sufferers according to the ICHD-II. The study population was homogenous, young students, and no major differences were found between the baseline characteristics of the two groups, other than a higher percentage of women among migraine sufferers (83.2%), a finding that is consistent with the epidemiological medical literature.[12]
Alcohol consumption and binge drinking prevalence in our study population was lower than reported in other large-scale studies among college students in America (which reported almost 44% of the students as being binge drinkers),[13] but similar to previously reported among Israeli students.[14]
When comparing hangover-proneness using the HSS between migraine sufferers and nonsufferers, it seems that migraine sufferers had a higher tendency to suffer from hangover on the first occasions they have consumed alcohol. This tendency attenuated through the years, and during the last year they have a similar tendency as compared with nonmigraine sufferers. Nevertheless, the tendency was higher to develop migraine-like symptoms of the hangover.
Migraine sufferers also show different drinking habits – they consume less alcohol, especially beer and liquors. Similar findings were found in other studies.[1516] It is possible, that due to higher tendency to develop hangover, “experienced” migraine sufferers voluntarily reduce their alcohol consumption, to avoid unpleasant hangover experience.
Surprisingly migraine sufferers consumed the same amount of wine as nonsufferers. Red wine was considered the most important trigger after the finding that 300 ml of red wine and not vodka with an equivalent alcohol content provoked headache in red wine-sensitive-migraine patients and not in nonsensitive migraine-patients or controls. Nevertheless, the headache triggered by red wine is not hangover. The interval between drinking red wine and developing headache varied from 30 min to 3 h, and only one or two glasses needed to be ingested.[8]
The present study was designed as a pilot study. Its main limitation is the fact that its data is based solely on self-reported questionnaires. Migraine diagnosis was based on these questionnaires and the students were not examined by a neurologist. A recall bias may be present regarding hangover symptomatology during the last year.
The finding that the tendency to develop migraine attacks affects the hangover symptomatology may suggest a similarity in pathophysiology, and possibly in treatment options.
Though the mechanism of alcoholic hangover is not fully understood, several physiological factors are likely to be contributory. These include the direct physiological effects of alcohol on the brain and other organs; withdrawal effect of alcohol; the physiological effects of alcoholic metabolites; the toxic effects of other active chemicals, like congeners, in the beverage; other behaviors associated with alcohol consumption, and certain personal characteristics; sleep disturbances and dehydration.[110]
The pathophysiology of migraine is still unclear and is continued to be researched. The past century has brought the shift from the vascular theory (that cranial vessels are the prime movers of the disorder) to a more integrated neurovascular theory, which takes the view that vascular change is secondary to neural activation (cortical spreading depression theory).[1718]
Perhaps inflammatory mechanisms and the known vasodilatatoric effect of alcohol are the link between hangover and migraine.
Only a few clinical trials have studied treatment for hangover symptoms. Some of the agents tested were propranolol, tropisetron, tolfenamic acid, fructose or glucose, and the dietary supplements Borago officinalis (borage), Cynara scolymus (artichoke), Opuntia ficus-indica (prickly pear), and a yeast-based preparation. There is no compelling evidence to suggest that any intervention, aside from abstinence or moderation, is effective for treating or preventing hangover symptoms.[19] Yet only small scale therapeutic trials were conducted on hangover treatments, and all of them were not powered to address different symptoms, but rather the symptom cluster as a whole. Future studies using specific antimigraine drugs such as triptans, may show efficacy in controlling at least part of the hangover symptoms cluster.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64-9.
- [Google Scholar]
- Epidemiology and impact of headache. In: Silberstein SD, Lipton RB, Dalessio DJ, eds. Wolff's headache and other head pain (7th ed). New York: Oxford University Press; 2001. p. :85-107.
- [Google Scholar]
- Migraine heterogeneity. Disability, pain intensity, and attack frequency and duration. Neurology. 1994;44(6 Suppl 4):S24-39.
- [Google Scholar]
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9-160.
- [Google Scholar]
- Migraine symptoms: Results of a survey of self-reported migraineurs. Headache. 1995;35:387-96.
- [Google Scholar]
- Alcohol and migraine: Trigger factor, consumption, mechanisms. A review. J Headache Pain. 2008;9:19-27.
- [Google Scholar]
- Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of hangover symptoms in college students. Alcohol Clin Exp Res. 2003;27:1442-50.
- [Google Scholar]
- Alcohol hangover: Mechanisms and mediators. Alcohol Health Res World. 1998;22:54-60.
- [Google Scholar]
- Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia. 1999;19:107-74.
- [Google Scholar]
- The epidemiology, burden, and comorbidities of migraine. Neurol Clin. 2009;27:321-34.
- [Google Scholar]
- Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. JAMA. 1994;272:1672-7.
- [Google Scholar]
- Survey of attitudes and use of psychoactive drugs among students in institutes of higher learning in Israel – 2003. 2003. Israel Anti Drug Authority. Available from: http://www.antidrugs.gov.il/download/files
- [Google Scholar]
- Cardiovascular risk factors and migraine: The GEM population-based study. Neurology. 2005;64:614-20.
- [Google Scholar]
- Current opinions on migraine pathophysiology. Neurol Neurochir Pol. 2004;38:307-15.
- [Google Scholar]
- Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24(Suppl 2):2-7.
- [Google Scholar]
- Interventions for preventing or treating alcohol hangover: Systematic review of randomized controlled trials. BMJ. 2005;331:1515-8.
- [Google Scholar]






