Translate this page into:
Acute neuromuscular weakness associated with dengue infection
Address for correspondence: Dr. HS Hira, House 74, Block 21, Lodi Colony, New Delhi - 110 003, India. E-mail: drhshira@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Dengue infections may present with neurological complications. Whether these are due to neuromuscular disease or electrolyte imbalance is unclear.
Materials and Methods:
Eighty-eight patients of dengue fever required hospitalization during epidemic in year 2010. Twelve of them presented with acute neuromuscular weakness. We enrolled them for study. Diagnosis of dengue infection based on clinical profile of patients, positive serum IgM ELISA, NS1 antigen, and sero-typing. Complete hemogram, kidney and liver functions, serum electrolytes, and creatine phosphokinase (CPK) were tested. In addition, two patients underwent nerve conduction velocity (NCV) test and electromyography.
Results:
Twelve patients were included in the present study. Their age was between 18 and 34 years. Fever, myalgia, and motor weakness of limbs were most common presenting symptoms. Motor weakness developed on 2nd to 4th day of illness in 11 of 12 patients. In one patient, it developed on 10th day of illness. Ten of 12 showed hypokalemia. One was of Guillain-Barré syndrome and other suffered from myositis; they underwent NCV and electromyography. Serum CPK and SGOT raised in 8 out of 12 patients. CPK of patient of myositis was 5098 IU. All of 12 patients had thrombocytopenia. WBC was in normal range. Dengue virus was isolated in three patients, and it was of serotype 1. CSF was normal in all. Within 24 hours, those with hypokalemia recovered by potassium correction.
Conclusions:
It was concluded that the dengue virus infection led to acute neuromuscular weakness because of hypokalemia, myositis, and Guillain-Barré syndrome. It was suggested to look for presence of hypokalemia in such patients.
Keywords
Dengue infection
hypokalemia
motor weakness
Introduction
Dengue infection is an endemic in South-East Asia. Four antigenically distinct dengue virus serotypes may cause the illness. Acute neuromuscular weakness may occur due to dengue infection. Dengue infection can cause myositis,[12] Guillain-Barré syndrome,[3] and hypokalemia.[4] However, there is paucity of literature documenting association between motor weakness and dengue infection.
Materials and Methods
The 88 dengue patients required hospital admission during the epidemic of dengue fever during the spring season of year 2010. The 12 patients (10.56%) developed motor weakness of the lower and/or upper limbs. We performed detailed clinical history taking and physical examination. None of them had reasons like drugs intake, intravenous fluid therapy, or gastro-intestinal loss to cause hypokalemia.
Serum potassium concentration <3.50 mmol/L established diagnosis of hypokalemia. The potassium level of 2.50 to 3.0 mEq/L was moderate hypokalemia. Severe hypokalemia was present when its level was < 2.50 mEq/L.
Apart from clinical profile, positive IgM and/or positive NS1 antigen (using standard kits for ELISA) substantiated the presence of dengue infection. We preserved serum at temperature of –70°C for detection of NS1 antigen and for virus serotyping by polymerase chain reaction. Dengue-specific IgM antibody was detected by μcapture ELISA (NIV, Pune). Panbio Early NS1 ELISA (Panbio, Australia) which is an NS1 antigen capture ELISA detected NS 1 antigen. Dengue serotyping was done by a multiplex RT-PCR method.[5]
Complete blood count, kidney and liver functions tests, serum electrolytes and serum creatine phosphokinase (CPK) were measured. Work-up of patients also included arterial blood gases (ABG) analysis. We did lumbar puncture in two patients who did not respond to potassium supplementation. In addition, both patients underwent nerve conduction velocity (NCV) and electromyography (EMG) in institutional neuoro-physiology department. Other causes of acute neuromuscular failure including diphtheria, poliomyelitis, Japanese encephalitis, vasculitis, myasthenia, botulism, and transverse myelitis were not present.
Patients with hypokalemia were infused with 40 mEq of potassium dissolved in 500 ml of 5% dextrose. In addition, we gave oral supplementation of potassium. One of us observed and monitored the recovery of weakness. Recording of breath counting monitored the respiratory muscle weakness.
Results
The age of 12 patients ranged between 18 and 34 years. All of them were male. Fever and neuromuscular weakness (quadriparesis) were the presenting symptoms in them. Motor weakness developed between 2nd and 4th day of onset of fever. One patient presented with motor weakness on the 10th day of fever. Oral mucosal bleed was present in three patients and platelet count was ≤ 43000 mm3 in them. Two patients showed self limited hypotension. On neurological evaluation, all patients had quadriparesis showing power <3/5. They had hypotonia as well as weak reflexes. None of them had sensory impairment. The urinary bladder and bowel functions were normal. Breath counting revealed no evidence of respiratory muscle weakness.
Ten (8.80%) patients had serum potassium level <3.0 mEq/L. Hypokalemia was mild in three, moderate in five, and severe in two patients. Level of sodium was normal in all of them. NCV suggested demyelinating pattern of Gullain-Barre Syndrome (GBS) in one patient and in others it was normal. EMG revealed myopathic pattern suggestive of myositis in single patient. Serum CPK and SGOT were higher in eight of 12 patients. Range of serum level of CPK was 250 U/L to 700 U/L in seven patients of hypokalemia while it was 5098 U/L in the patient of myositis [Table 1]. CPK levels were <250 U/L in three subjects. Their SGOT levels were mildly elevated. ABG analysis did not show disturbance. The CSF investigations were normal in patients of GBS and myositis. All patients showed thrombocytopenia ≤900000/mm3 during the course of the illness. Nine patients had leucopenia (<4,500/mm3). ECG did not reveal any abnormality.
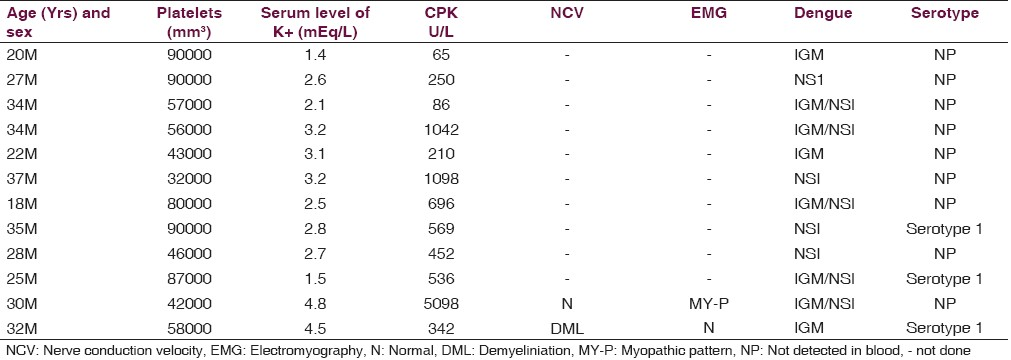
Five patients were positive for NS1, among them one was of myositis. In five patients, both IgM dengue serology and NS1 antigen were present. Dengue virus was isolated in three patients belonging to serotype 1.
Patients with hypokalemia were infused with 40 mEq of potassium dissolved in 500 ml of 5% dextrose. Weakness responded promptly in 6 to 12 hours. All of them were able to walk following day. There was no recurrence of muscle weakness subsequently. Both muscle tone and tendon reflexes became normal. No patient needed mechanical ventilation, because respiratory muscle weakness did not develop in any patient.
Potassium level of patient of myositis was normal (>3.5 mEq/L) throughout the illness, and he showed recovery though it was slow. The patient with diagnosis of Guillain-Barré syndrome also showed normal potassium level; he improved within 6 weeks without any sequel.
Discussion
Acute onset of neuromuscular weakness complicating dengue infection is not unknown.[1–4] However, its mechanism is still not clear. One study reported that seven patients of neuromuscular weakness afflicted with dengue infection demonstrated the high serum levels of CPK but their EMG was normal; however, the histopathology of their muscle biopsy revealed myositis.[1] Other study reported seven cases of dengue myositis and their CPK levels were high i.e. 16,000 to 120,000 U/L.[2] They recovered completely within 4 weeks. Report of Guillain-Barré syndrome complicating dengue infection was available.[3] Three patients of dengue fever had acute pure motor quadriparesis due to hypokalemia, however, motor weakness reversed in them with potassium supplementation.[46] The reason for the development of hypokalemia was due to intravenous fluid therapy in that study. Hypokalemic paralysis in Chikungunya fever had also been reported.[7]
In present study, 10 of 12 patients of neuromuscular weakness showed low serum potassium level. Within a short period, all of them had complete and prompt recovery with potassium correction without any sequel. These patients did not have any obvious factors to cause hypokalemia e.g. consumption of drugs (diuretics), intravenous fluid intake and gastro-intestinal loss. Usage of intravenous fluid especially lactate-containing solutions may promote metabolic alkalosis in the body and potassium shifts intra-cellular, thus results in low level of potassium. Authors agreed with proposal put forth for mechanism of genesis of hypokalemia by Jha and Ansari[4] who suggested that hypokalemia could be either due to redistribution of potassium in cells or transient renal tubular abnormalities leading to increased urinary potassium wasting. In addition, we assumed that stress induced catecholamine release might had induced cellular uptake of potassium to result in hypokalemia patients of our study.
In severe hypokalemia, muscle necrosis can occur, and at serum concentrations of potassium less than 2.0 mmol per liter, an ascending paralysis can develop with eventual impairment of respiratory functions.[8] No patient developed respiratory muscle weakness requiring mechanical ventilation in this study. One study suggested that the breakdown of tissues during infectious diseases releases potassium into the extracellular compartment, mitigating hypokalemia.[9]
The extent of level of potassium showed no relation to the presence of IgM, NSI, and virus serotype. In this study, CPK level of a patient of myositis was markedly raised and his EMG showed myopathic pattern. The pathogenesis of myositis may incriminate myotoxic cytokines, particularly tumor necrosis factor (TNF).[10] The NCV was suggestive of demyelinating peripheral nerve disease in a case of Guillain-Barré syndrome; and patient had complete recovery over 6 weeks. The proposed mechanism for Guillain-Barré syndrome is that an antecedent infection evokes an immune response, which in turn can affect the myelin or the axon of peripheral nerve.[11] The level of potassium was normal in both patients of GBS and myositis. Six children with dengue encephalitis on the second or third day of illness were reported.[12] Postulation was that the virus crosses the blood-brain barrier and directly invades the brain causing encephalitis. Dengue patients presenting with encephalopathy had more severe illness and worse outcome compared to acute pure motor weakness.[13]
Dengue virus of serotype1 was isolated in two patients of hypokalemia and in single case of myositis in our study [Table 1].
To conclude, the hypokalemia among patients of dengue was one of the important causes of acute motor weakness. Clinicians should be aware of such association while evaluating the case of fever of dengue infection. In countries endemic to dengue, it will be prudent to investigate patients of dengue infection with neurological manifestations. There is also need to understand the pathogenesis of the neurological manifestations related to the dengue infection.
Source of Support: No support in form of funds and equipment.
Conflict of Interest: No conflict of interest among authors.
References
- Acute pure motor quadriplegia: Is it dengue myositis? Electomyogr Clin Neurophysiol. 2005;45:357-61.
- [Google Scholar]
- Acute dengue virus myositis: A report of seven patients of varying clinical severity including two cases with severe fulminant myositis. J Neurol Sci. 2011;300:14-8.
- [Google Scholar]
- Guillain Barre syndrome in the course of dengue: case report. Arq Neuropsiquiatr. 2004;62:144-6.
- [Google Scholar]
- Dengue infection causing acute hypokalemic quadriparesis. Neurol India. 2010;58:592-4.
- [Google Scholar]
- Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol. 1998;36:2634-9.
- [Google Scholar]
- The prevalence of hypokalemia in hospitalized patients with infectious diseases problem at Cipto Mangunkusumo Hospital, Jakarta. Acta Med Indones-Indone. 2006;38:202-5.
- [Google Scholar]
- Hypokalemic paralysis following Chikungunya fever. J Assoc Physicians India. 2007;55:598.
- [Google Scholar]
- A survey of hypokalemia in patients of general practitioners. Br J Clin Pract. 1988;42:192-5.
- [Google Scholar]
- Neurological manifestations of dengue virus infection. J Neurol Sci. 2006;244:117-22.
- [Google Scholar]






