Translate this page into:
Acute Hemiplegia in Children: A Prospective Study of Etiology, Clinical Presentation, and Outcome from Western India
Address for correspondence: Dr. Amitabh Singh, Department of Pediatrics, Chacha Nehru Bal Chikitsalaya, New Delhi - 110 031, India. E-mail: dramit_amy@yahoo.co.in
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Hemiplegia/hemiparesis denotes the weakness of one side of the body. In contrast to adults, hemiparesis in children occurs secondary to a variety of etiological conditions.
Aims:
The aim of this study was to assess the clinical, laboratory, and radiological features of children with acquired hemiparesis/hemiplegia of nontraumatic origin and intended to find its underlying etiology in the Indian children.
Settings and Design:
This prospective, observational study was carried out at a tertiary care hospital in western India.
Materials and Methods:
Children aged between 3 months and 14 years admitted to the in-patient department of a tertiary care hospital with acquired hemiparesis/hemiplegia were included over 2 years. Children with perinatal insult, preexisting neurological diseases, neurotrauma, hemiplegic migraine, and Todd's paralysis were excluded from the study. Detailed clinical examination, laboratory, and radiological investigations were done, and an attempt was made to find the underlying etiology. These children were also followed up after 1 month of discharge to look at short-term outcomes. All clinical information was recorded in a predesigned performa and was managed with Microsoft Excel spreadsheet. Frequency was presented as number (N) and percentage (%).
Results:
Fifty-five children (male:female = 1.2:1), predominantly between 1 and 5 years of age were studied. Apart from weakness (92.8%), vomiting (70.9%), fever (58.2%), and seizure (58.2%) were the predominant presenting complaints. One-fifth of them had comorbidities; most commonly congenital heart disease. Cerebral infarction was the most common pathology in neuroimaging. Central nervous system infection (45.5%) was the most common identified etiology followed by vascular events (21.8%). Among those who could be followed up at 1 month, about 65% had some improvement in their power.
Conclusion:
Infections continue to be an important cause of neurodisability in the developing countries.
Keywords
Central nervous system infection
cerebral infarction
stroke
INTRODUCTION
Hemiplegia refers to a total paralysis of the arm, leg, and trunk on one side of the body, while hemiparesis is a muscle weakness (or partial paralysis) of one side of the body. Although not identical, both these terms have been used interchangeably in the following text unless specified otherwise. It is not a disease, but rather a nonspecific response of the central nervous system (CNS) to a range of insults.[12] Hemiparesis/hemiplegia is less common in children compared to adults, but the pathological condition and underlying etiologies thereof are much more varied.[12] In adults, the most common cause of hemiparesis is a cerebrovascular accident or stroke. In children, on the other hand, a number of other conditions apart from stroke can present as hemiparesis including but not limited to CNS infection (e.g., encephalitis, meningitis, and abscess), neoplastic intracranial space-occupying lesions (ICSOL), trauma, and developmental anomalies of the brain.[1234] During the latter part of 19th century, Freud described “acute hemiplegia of childhood” as “A previously healthy child without hereditary predisposition suddenly becomes ill from a few months to 3 years of age. The etiology of the illness remains unknown or is sought in a simultaneously occurring infectious disease”[5] With the advent of more sophisticated investigations modalities such as cerebral angiography and fewer etiologies remained “unknown.”[2] However, even after a century, there have been only a handful of studies looking at the clinic-radiological features and etiological profile of hemiplegia/hemiparesis in pediatric population.[3468910] Most of these studies again included children with congenital weakness secondary to perinatal insults with only a few[4] exploring specifically the acquired ones.
This study was carried out to assess clinical, laboratory, and radiological features of children with acquired hemiparesis/hemiplegia of nontraumatic origin and intended to identify underlying etiology in the children presenting to the pediatric department at a regional apex tertiary care public healthcare institution.
MATERIALS AND METHODS
This prospective observational study was carried out in the Department of Pediatrics of a tertiary care hospital of Western India from November 2010 to October 2012 after obtaining clearance from the Institutional Ethics Committee. Children aged between 3 months and 14 years admitted to the inpatient department (IPD) with hemiparesis or those who developed hemiplegia during their stay in the hospital were included in the study after obtaining informed written consent from their parents/legal guardians. Hemiparesis was defined as having a score of 4 or less as graded by the Medical Research Council (MRC) scale[11] in the affected upper or lower limb in either proximal or distal muscle groups. Children with diagnosed cerebral palsy/birth asphyxia (Apgar score ≤5 at 10 min or pH <7.0, or base deficit ≥16 in cord blood or arterial blood sample obtained within 1 h of birth), history of hyperbilirubinemia requiring exchange transfusion, preexisting neurological diseases, neurotrauma, hemiplegic migraine, and Todd's paralysis were excluded from the study. A detailed structured medical history was documented, and meticulous clinical examination was done in all study patients. All patients included in this study underwent an initial panel of investigations which included as follows: complete blood counts, coagulation profile (prothrombin time [PT], activated partial thromboplastin time [APTT]), renal and liver function tests, fundus examination, Mantoux test, chest X-ray, and neuroimaging (contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging [MRI] of brain). Further investigations were performed based on the diagnostic clues obtained thereof.
In cases, hemorrhagic stroke was identified, CT/MRI angiography ± MR venography was performed. When an ischemic stroke was identified, an ultrasound of the cervical arteries, CT/MRI angiography, electrocardiogram, and echocardiography were performed.
Cerebrospinal fluid (CSF) analysis was done in those with suspected CNS infection based on clinical features and/or neuroimaging after ruling out raised intracranial tension. Analysis included total and differential cell count, CSF biochemistry, bacterial culture, herpes simplex virus polymerase chain reaction (PCR), Japanese encephalitis virus PCR, acid-fast bacilli, and mycobacterium growth indicator tube culture. A diagnosis of pyogenic meningitis was made in the presence of CSF pleocytosis (>1000/mm3) with polymorphonuclear leukocytosis, hypoglycorrhachia (CSF sugar <50% of blood sugar), increased protein, and a positive Gram stain/bacterial culture.[12] The diagnosis of tubercular meningitis (TBM) was based on the clinical case definition by Doerr et al.[13]
Definition/criteria used to define the following were:
Anemia – low blood hemoglobin levels according to the age-related cutoffs given by the World Health Organization;[14] Coagulopathy – increased PT and/or APTT according to the cutoffs provided by the manufacturer; Fully immunized – a child who had completed the recommended schedule of BCG, DPT, and OPV (three doses) and measles vaccine before 1 year of age;[15] Partially immunized – a child who had received some immunizations but did not satisfy the definition of fully immunized; not immunized – a child who had not received any vaccine until date; significant lymphadenopathy – enlarged inguinal lymph nodes >1.5 cm and >1 cm at other sites; and positive Mantoux test – an induration >10 mm measured across the long axis of the forearm.[16]
Patients were treated according to the standard institutional guidelines (medical ± surgical), and the researchers did not have any influence on the same. The children were followed up between 7 and 10 days after discharge and at 1 month. At each follow-up visit, a thorough neurological examination was done. A clinical improvement was considered to be present if there was an improvement in power by ≥1 MRC grade at any of the muscle groups.
All clinical information was recorded in a predesigned performa and was managed with Microsoft Excel spreadsheet. Frequency was presented as number (N) and percentage (%).
RESULTS
A total of 12438 children were admitted to the IPD during the study period; of them 68 (0.55%) patients had hemiparesis. Thirteen children were excluded as they met exclusion criteria, and the other 55 were included in this study [Figure 1].
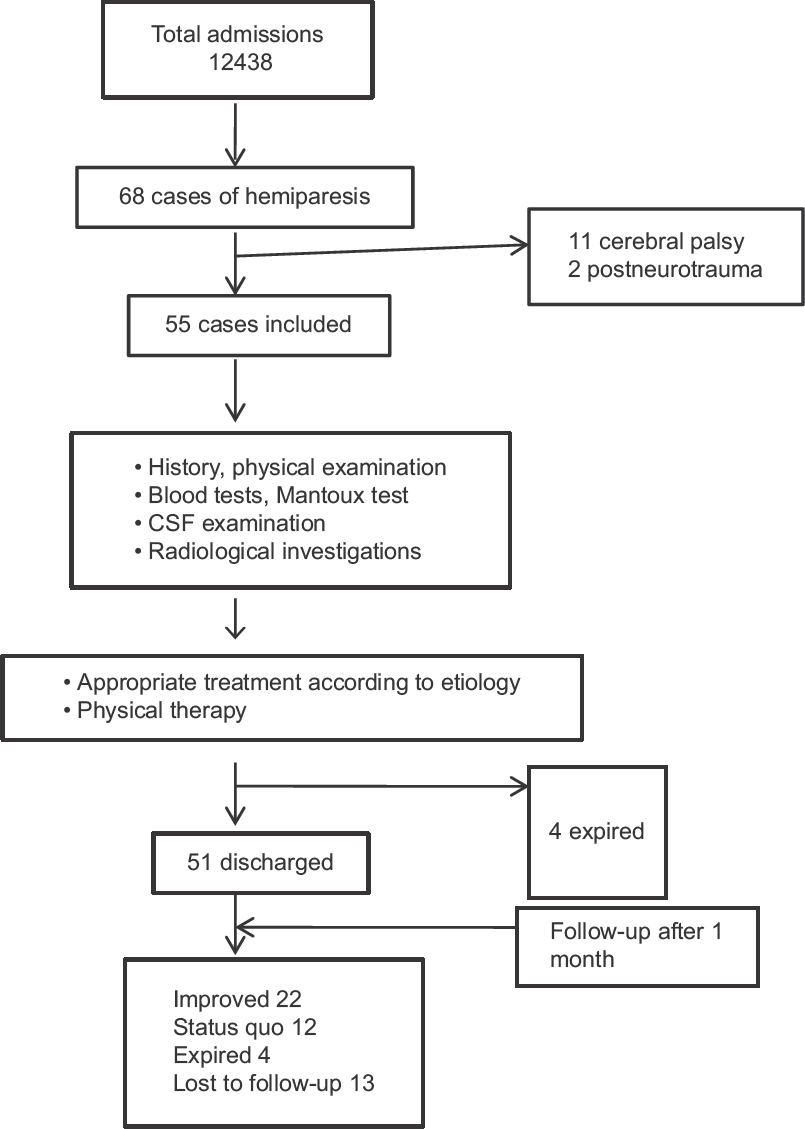
- Study flow
Most (60%) of the children were within 2–5 years of age and a slight male predominance (male: female = 1.2:1) was noted. The most common symptom was unilateral weakness of limbs (92.8%), followed by vomiting (70.9%), fever (58.2%), and seizures (58.2%). Eleven (20%) children had preexisting comorbidities; congenital heart disease (CHD) (9.1%) was the most common, followed by TB (3.6%). The right side of the body was involved more commonly (56.4%) and isolated upper motor neuron type of facial palsy was observed in 45 (81.2%) cases. Of a total 23 (41.8%) children with an altered level of consciousness at presentation, four (7.3%) were comatose, and in almost half of the cases, (27 [49.9%]) signs of meningeal irritation could be elicited. Abnormal findings of cardiovascular system examination in the form of a cardiac murmur were present in 9.1% of the cases, whereas 14 (25.4%) children each had an abnormal respiratory system examination and 2 (3.6%) had hepatosplenomegaly [Table 1].
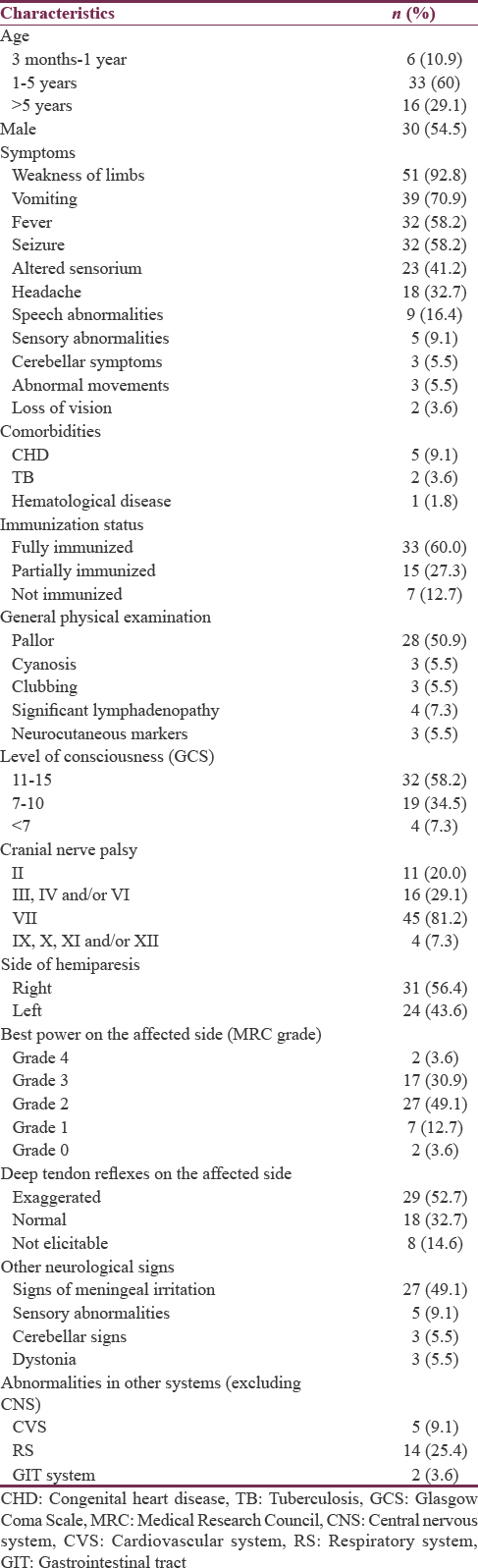
While investigating these children [Table 2], 58.2% were noted to have anemia, with all of them having microcytic and hypochromic red blood cells. Echocardiography revealed congenital cyanotic heart disease (CCHD) (Fallot physiology) in three, whereas, two children had ostium secundum (OS) type atrial septal defect (ASD) with a bidirectional shunt. Electroencephalogram was abnormal in 16 (four children were comatose and had diffuse slowing while 12 others had focal discharges). Chest radiographs were abnormal in 12 children including two patients with one each having consolidation with mild pleural effusion and miliary lesions, respectively, suggestive of pulmonary tuberculosis. Neuroimaging revealed cerebral infarction in 30 (54.5%) cases, while an ICSOL was postulated to be the primary cause of hemiparesis in a further 12 children (21.8%).
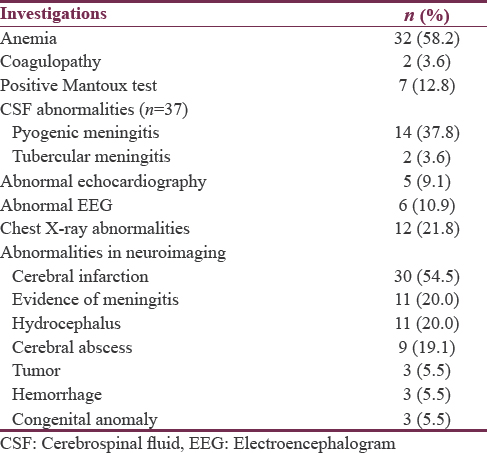
An etiological diagnosis could be ascertained in 81.8% of cases with infection being the most common cause (45.5%) followed by vascular (21.8%) [Table 3].

Only, 34 children could be followed up until 1 month; among them 22 had an improvement in their muscle power, whereas in 12, there was no improvement [Figure 1]. None of them had worsening of their weakness or recurrence of seizure during this period.
DISCUSSION
Fifty-five children with acquired (nontraumatic) hemiparesis aged between 3 months and 14 years admitted at a tertiary care center in Western India were studied by the authors to look into their clinic-radiological features and also to find out the underlying etiologies.
Hemiparesis was seen in only 0.44% of all pediatric admissions during the study period with a slight male preponderance. There is no similar previous study reporting the prevalence of hemiparesis in hospital-admitted children although a similar male predominance was observed in previous studies from India;[39] it should be interpreted cautiously as it could simply reflect a referral bias. Most of the children were between 1 and 5 years of age, possibly due to an increased incidence of infections at this age leading to hemiparesis directly or indirectly by cerebrovascular accidents.[17] Vomiting was the most common associated symptom seen in about 71% of cases; fever and seizure were present in about 60% followed by unconsciousness and headache. In a previous similar study by Raghu Raman et al.,[3] also unconsciousness, fever, vomiting, and headache were found to be the most frequent complaints. The same has been echoed in the study by Shivalli et al.[9] These symptoms were possibly attributed to raised intracranial pressure and cerebral irritability associated with the intracranial pathology.
Comorbidities were present in one-fifth of the patients. Of the five children with CHD, the ones with CCHD had venous thrombosis while the two children with an OS ASD were found to have an arterial ischemic stroke. The relationship between CCHD and CVA has been well documented with postulated mechanisms such as hyperviscosity, relative anemia, hypoxemia, and iron deficiency.[18] However, interestingly, none of the cases of cerebral abscess were related to cyanotic heart disease which is another cause of hemiparesis in such patients.[19] On the other hand, the relationship of CVA with ASD is controversial.[20] However, it is undeniable that these children are at a risk of recurrence of stroke and need appropriate long-term care.[21] Both children with TB had dissemination of their disease to brain leading to possible arteriopathy and acute ischemic stroke. TBM is a known cause of CVA[22] and has been an important cause of hemiparesis in children.[3] The one child with coagulopathy had hemophilia and developed intracerebral hemorrhage.
CNS infections comprising of pyogenic/TBM and the cerebral abscess was the predominant cause of hemiplegia in the studied children. Local vasculitis and thrombosis secondary to CNS infection are the implicated mechanism leading to hemiplegia.[23] Since the first description by Freud, infections have always been an important cause of hemiparesis in children over the years accounting for 15%–56% of cases.[34917] Not only acute neuroinfections but also preceding infections are also known to increase the risks of stroke in children.[24]
Among the vascular causes of hemiparesis moyamoya disease (9.1%) was the most common, followed by cerebral sinus venous thrombosis (CSVT) (7.3%) and arteriovenous malformation (3.6%). The cases of moyamoya pattern on MR angiography were not divided into moyamoya disease (primary) or moyamoya syndrome (secondary) in this study. Before the introduction of noninvasive neuroimaging techniques, in a retrospective autopsy study of 555 cases, occlusive cerebrovascular disease was observed in 44 (8.7%).[25] Solomon et al. in their series of 86 children[4] found that 10 patients of 36 who had undergone arteriography had basal vascular occlusive disease either with or without telangiectasia. Three children had stenosis at the origin of the internal carotid artery, two had distal branch occlusion and one had polyarteritis nodosa. Newer studies, on the other hand, have documented a prevalence of 20%–30% of vascular etiology in childhood hemiparesis.[37] Isler in his comprehensive review of angiographic studies in 116 cases of acute infantile hemiplegia emphasized that even a normal arteriogram did not exclude the possibility of a vascular etiology due to the importance of the timing of the arteriogram.[26]
Solomon et al., in their review, tried to look at the risk factors for long-term complications of hemiplegia in children.[4] They concluded that the onset of hemiplegia associated with seizures in children with occlusive vascular disease predicted subsequent epilepsy and a persistent motor deficit. Children presenting without seizures had a small risk (17%) of later developing seizures.
Furthermore, intellectual deficits were more likely to occur if the child aged <2 years at presentation and had fever and multiple seizures to begin with. With a very short follow-up, we could not assess such long-term outcomes or risk factors thereof. It may be noted however that apart from four children who died, the ones turning up at 1 month of follow-up did not have any deterioration of weakness.
Some limitations were noted and should be acknowledged in this study. First, this study population being tertiary care center based, referral bias cannot be ruled out. Second, we could not perform any tests for hypercoagulable states such as protein C, protein S, serum homocysteine levels in children with cerebral infarction, and CSVT. Although, the utility of these tests are questionable in the acute setting they certainly decide the long-term management and prognosis. Third, children with ischemic stroke were not tested for infectious agents such as mycoplasma and chlamydia, as well as enterovirus, parvovirus 19, influenza A, Coxsackie, Rocky Mountain spotted fever, cat scratch disease, etc., that are known to predispose the same.[23] Fourth, we also did not test the children for metabolic conditions which are mimickers of stroke such as mitochondrial encephalopathies and methylmalonic acidemia.[2] Finally, the duration of follow-up was very short with a very high attrition rate and did not permit more long-term assessment of outcomes in children with hemiparesis included in this study.
CONCLUSION
Hemiparesis/hemiplegia though rare in children is a cause of significant mortality and morbidity. Infections continue to be important cause of neurodeficit, at least in the developing countries. Studies with long-term follow-up of children with hemiparesis, especially those secondary to vascular and cardiac etiologies are required to gain further knowledge about their natural history and subsequent risk of recurrence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Hemiplegia. In: Clinical Pediatric Neurology: A Signs and Symptoms Approach (6th ed). Philadelphia, PA: Saunders/Elsevier; 2009. p. :248-65.
- [Google Scholar]
- Acute childhood hemiplegia. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:1244.
- [Google Scholar]
- Clinical analysis of pediatrichemiplegia at tertiary care hospital in Bangalore. Int J Basic Appl Med Sci. 2014;4:246-57.
- [Google Scholar]
- Medical Research Council. Aids to the Investigation of the Peripheral Nervous System. London: Her Majesty's Stationary Office, Medical Research Council; 1943.
- [Google Scholar]
- Clinical and public health aspects of tuberculous meningitis in children. J Pediatr. 1995;127:27-33.
- [Google Scholar]
- World Health Organization. Haemoglobin levels to diagnose anaemia at sea level. In: Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: WHO; 2001. p. :3.
- [Google Scholar]
- Missed opportunities for immunization in children under 2 years attending an urban teaching hospital. Indian Pediatr. 1995;32:51-7.
- [Google Scholar]
- WHO Guidelines Approved by the Guidelines Review Committee. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children. (2nd ed). Geneva: World Health Organization; 2014.
- [Google Scholar]
- Etiology of strokes and hemiplegia in children presenting at Ayub Teaching Hospital, Abbottabad. J Ayub Med Coll Abbottabad. 2006;18:60-3.
- [Google Scholar]
- Cerebrovascular accidents in infants and children with congenital cyanotic heart disease. Isr J Med Sci. 1984;20:1143-5.
- [Google Scholar]
- Cerebral abscess in patients with congenital cyanotic heart disease. Neurol India. 1970;18(Suppl 1):96-9.
- [Google Scholar]
- Stroke recurrence in children with congenital heart disease. Ann Neurol. 2012;72:103-11.
- [Google Scholar]
- Preceding infection as a risk factor of strokes in the young. J Assoc Phys India. 1999;47:673-5.
- [Google Scholar]
- Cerebral vascular disease in infancy and childhood 1. Occlusive vascular diseases. J Neuropathol Exp Neurol. 1961;20:127-40.
- [Google Scholar]
- Acute Hemiplegia and Hemisyndromes in Childhood. Clinics in Developmental Medicine, Nos 41/42. London: Spastics International Medical Publications, William Heinemarm Medical Books Ltd; 1971. p. :3-313.
- [Google Scholar]






