Translate this page into:
Acute Febrile Encephalopathy in Children: A Prospective Study of Clinical Features, Etiology, Mortality, and Risk Factors from Western India
Address for correspondence: Dr. Charul S. Purani, Department of Pediatrics, B. J. Medical College and Civil Hospital, Ahmedabad - 380 016, Gujarat, India. E-mail: charulpurani@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Acute febrile encephalopathy (AFE) in children is a medical emergency and could be a manifestation of many systemic and central nervous system pathologies. The clinical features of AFE are nonspecific and etiological spectrum variable depending on the studied population.
Materials and Methods:
A prospective, observational study was carried out including children aged between 1 month and 12 years with AFE admitted to the Pediatric Intensive Care Unit of a tertiary care hospital in Western India. The primary objective was to assess the clinical presentation and etiology of AFE while the secondary objectives were to correlate the clinical and etiological findings and to determine the risk factors associated with mortality.
Results:
Out of the ninety children with AFE included in this study, male:female ratio was 1.2:1; most of them were aged between 1 and 5 years and came with a history of < 7 days (82.2%). All of them had altered sensorium, about 2/3rd had seizures and 47.8% having a Glasgow Coma Score (GCS) <8. Etiology remained elusive in about 40% of the cases, and viral infections were the most common among the ones with an identifiable cause. A variety of morbidity (shock, disseminated intravascular coagulopathy, respiratory failure, etc.) and high mortality (40%) was observed with risk factors associated with mortality being GCS < 8, the presence of raised intracranial pressure, shock, and respiratory failure.
Conclusion:
AFE, though a rare diagnosis in children, is associated with significant morbidity and high mortality in a developing country like India.
Keywords
Central nervous system infection
children
encephalitis
encephalopathy
mortality
seizure
INTRODUCTION
Encephalopathy is a nonspecific term, meaning “disease of the brain” in Greek. It is rather a clinical syndrome, with diverse etiopathologies encompassing various organ systems, extending well beyond central nervous system (CNS). A child presenting with fever, altered cognition or personality, and altered sensorium and/or seizure is labeled as acute febrile encephalopathy (AFE); it is a medical emergency as well as a diagnostic and therapeutic challenge for the pediatrician.[1] A subset of such patients with evidence of inflammation of brain parenchyma, either infectious or noninfectious, is called as encephalitis.[2] Limited literature in children suggests CNS infections to be the most common cause of AFE in India[3456] and other developing countries;[789] though there has been a great variation in the frequency of different contributing etiologies across the globe and even different regions within a country. The nonspecific nature of clinical features further makes the clinical prediction of possible etiology very difficult in cases of AFE; which in turn, may lead to a delay in institution of appropriate therapy. The paucity of data on risk factors associated with AFE in children is also well appreciated.[3]
This prospective, observational study was carried out in the Department of Pediatrics of a tertiary care hospital in Western India. The primary objective was to assess the clinical presentation and etiology of AFE in children between 1 month and 12 years while the secondary objectives were to correlate the clinical and etiological findings and to determine the factors associated with mortality.
MATERIALS AND METHODS
Children between 1 month and 12 years of age admitted to the Pediatric Intensive Care Unit (PICU) of B J Medical College, Ahmedabad, during the study period (October 2013 to October 2015) were included if they satisfied the case definition of AFE and did not meet the exclusion criteria. Informed written consent was obtained from parents/guardian, and ethical clearance for the study was taken from Institution Ethics Committee. Definitions used in the study included as follows:
-
Anemia-as per age-related cutoffs’ given by the WHO[10]
-
Leukocytosis-total leukocyte count >11,000/mm3
-
Leukopenia-total leukocyte count < 4000/mm3
-
Thrombocytopenia-platelet count < 150,000/mm3
-
Acute renal failure-as per acute kidney injury (AKI) network criteria[11]
-
Acute respiratory failure and DIC-standard definitions as per[12] and[13] respectively.
AFE was defined as acute onset (≤14 days) fever (axillary temperature >35.5°C or 95.5°F) with altered state of consciousness lasting for ≥12 h and/or seizure. Exclusion criteria were the duration of illness >14 days at presentation, treated outside before admission to our center, known chronic systemic illness including neurodevelopmental delay, malignancy, and immunosuppressive therapy. Children with metabolic encephalopathy, dyselectrolytemia (in the absence of any cerebrospinal fluid [CSF] abnormality), evidence of demyelination in neuroimaging, intracranial space occupying lesion, febrile seizure, endocrinal encephalopathy, and stroke, if subsequently diagnosed after investigation, were also excluded from the study.
Patient's demographic data and detailed history were recorded. Special mention was made to prodrome of the upper respiratory illness, flu-like illness or diarrhea; animal bite or recent vaccination; recent history of contact with a person having measles, chickenpox or mumps, recent history of travel, or any occurrence of similar illness in the neighborhood. Assessment of socioeconomic status was done according to the modified Kuppuswamy scale.[14] Clinical examination included vital parameters, anthropometry, general physical examination, and a detailed systemic examination with special emphasis on neurological examination. The nutritional status assessment was done according to IAP classification.[15] Clinical level of consciousness was assessed by modified Glasgow Coma Score (GCS).[16] A diagnosis of raised intracranial tension was made when two or more of the following were present: hypertension, bradycardia, irregular respiration, abnormal tonic posturing, bulging, and nonpulsatile anterior frontanel when they are open, presence of crack-pot sign, and papilledema on direct ophthalmoscopy when fontanel are closed.[17]
All the children underwent the following investigations: complete blood count, blood culture, random blood sugar, kidney function test, serum electrolytes, liver function test, malaria antigen test and peripheral smear (thin and thick) for malaria, NS1 antigen, and IgM antibody for dengue, serum IgM antibody for Japanese encephalitis (JE); CSF examination for biochemistry (protein, sugar), cytology, Gram stain, bacterial culture, genexpert for tuberculosis (TB), polymerase chain reaction for herpes simplex virus (HSV) and varicella zoster virus, IgM ELISA for JE virus; Mantoux test; chest X-ray, neuroimaging (ultrasonography [USG] of brain, contrast-enhanced computed tomography [CECT] of the brain and magnetic resonance imaging [MRI] of brain). Predefined criteria were used in the etiologic diagnosis of AFE [Table 1].
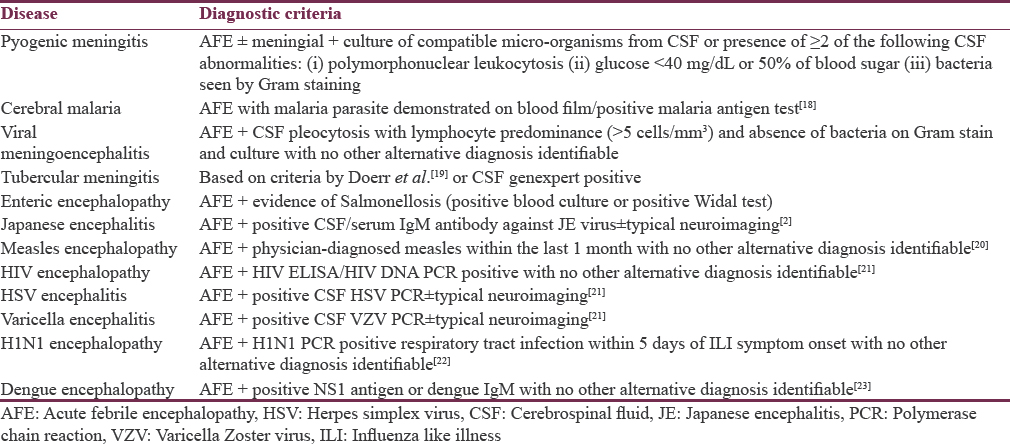
All the children received treatment based on standard management guidelines depending on the presumptive/established diagnosis, and they were assessed for morbidity/complications during hospital stay and outcome in the form of mortality or discharge from the hospital.
Data were collected on structured pro forma and managed on Microsoft Excel sheet. The analysis was done using Stata 10 software (STATA Corp, College Station, TX, USA). Chi-square test and Fisher's exact test were performed to test for differences in proportions of categorical variables between two or more groups.
RESULTS
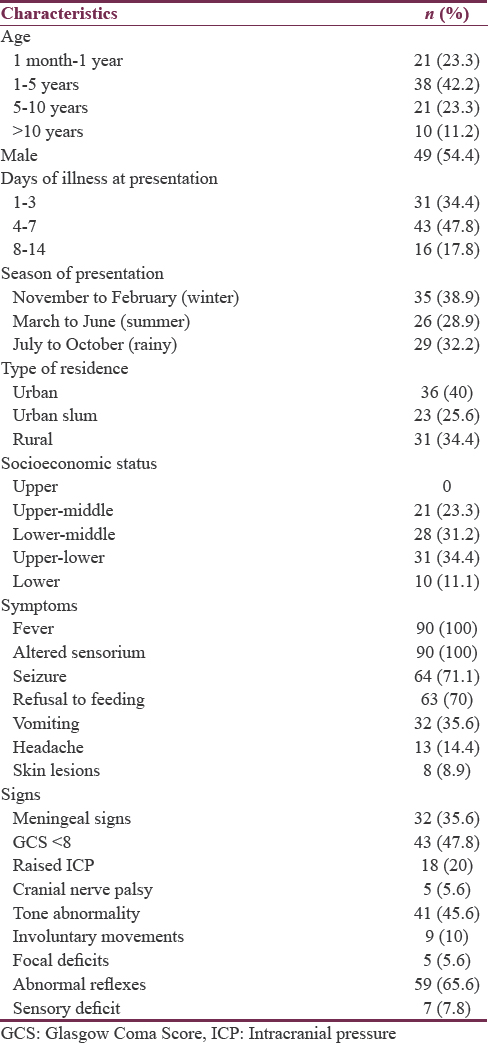
Out of the total 15,886 children admitted to the hospital during the study, 924 (5.8%) were admitted in PICU. Among the PICU admissions, 116 (12.6%) patients fulfilled the case definition of AFE,[16] of which 90 cases of AFE were included in the study as 26 met the exclusion criteria. The overall prevalence of AFE among hospitalized children was very small (0.7%). The baseline clinicoepidemiological characteristics of the study participants are presented in Table 2.
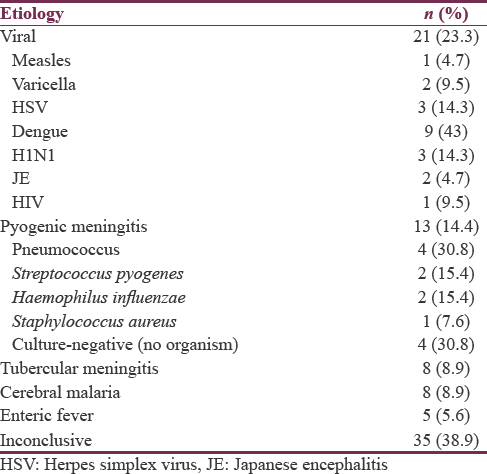
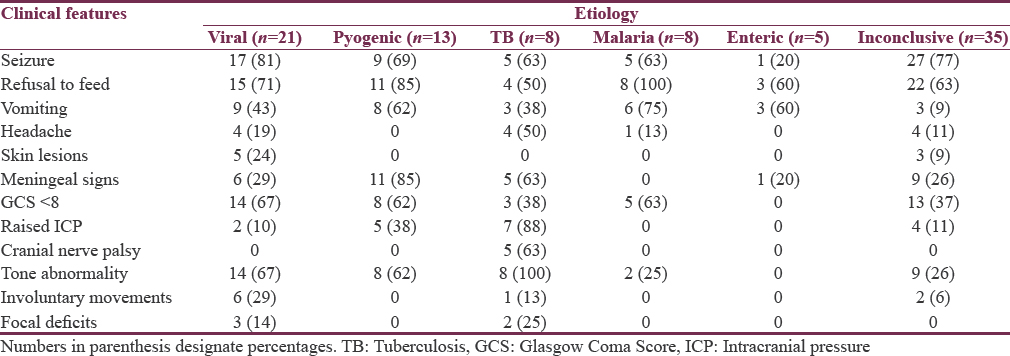
The most common etiology of AFE found in our study was viral encephalitis (23.3%) followed by pyogenic meningitis (14.4%); in about 39% cases no etiology could be ascertained [Table 3]. Table 4 presents the relative frequency of different clinical features in different etiological categories.
All the children underwent neuroimaging with one or more modalities (USG brain, CECT brain, and MRI brain). Among the USG brain done in infants with open anterior fontanel, 2 revealed features suggestive of communicating hydrocephalus (i.e. dilatation of all ventricles); in CECT brain, 13 were normal, 12 showed areas of infarct with hydrocephalus, and 10 had only cerebral edema but no features of herniation. Forty-one children had MRI brain (± contrast) done with 32 having an abnormality.
Anemia was observed in 58 (64.4%) cases; among them, 5 cases had severe anemia, all with cerebral malaria. Leukocytosis was much more common (42.2%) then leukopenia (20%) while thrombocytopenia was seen in 21 (23.3%) cases. Renal dysfunction (AKI Stage 1 and 2) were detected in 26 (28.8%) children, but none of them required renal replacement therapy. Dyselectrolytemia was present in 31 (34.4%) cases, the most common being hyponatremia (23/31). Twenty-one (23.3%) children had derangement of liver function, but none of them had icterus or evidence of acute liver failure.
Most common serious complication seen in the present study was shock (35.6%) and disseminated intravascular coagulation (32.2%), followed by acute respiratory failure (20%). Forty-three children required mechanical ventilation and 30 were treated with inotropes.
Thirty-six (40%) children with AFE expired and 1 child left against medical advice. Highest mortality rate was observed in the inconclusive etiology group (50%) followed by viral etiologies (25%) [Table 5].

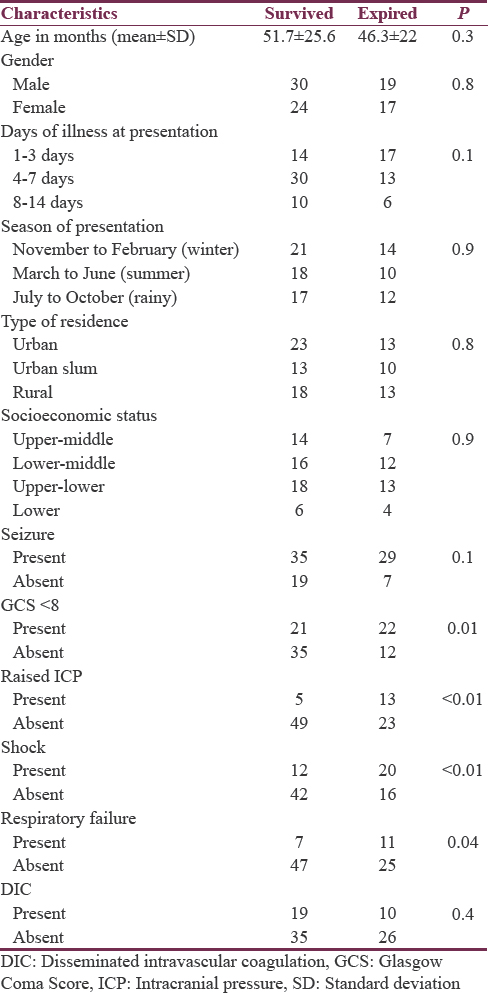
GCS < 8, the presence of raised intracranial pressure (ICP), shock, and respiratory failure were found to be risk factors associated with mortality [Table 6].
DISCUSSION
In this hospital-based prospective study, we analyzed clinical features, etiology, and outcome of 90 children admitted in PICU with AFE. AFE was a rare cause of hospital admission (0.7%) in children but constituted 12.6% of all PICU admissions. A variety of morbidity and very high mortality (40%) was observed with risk factors for mortality being GCS < 8, the presence of raised ICP, shock, and respiratory failure.
There was a slight male preponderance (male:female = 1.2:1) and majority of the patients belonged to the age group of 1 and 5 years (42.7%); most of the patients (82.2%) had presented within 1st week of illness. The previous Indian studies have almost always demonstrated a male preponderance, and the most commonly affected age group has also remained the same.[45] The time of presentation usually depends with the acuteness of the onset and also probably reflects the severity of illness evident by the fact that children presenting within the 1st week of symptoms had a lower GCS score and seizure compared to those presenting late (data not shown).
After fever (100%) and altered sensorium (100%), which are diagnostic criteria for AFE, seizure (71.1%), and refusal to feed (70%) were the major complaints, while abnormal reflexes and tone abnormalities were the most common abnormality on clinical examination present in around 65% and 45% of cases, respectively. Cases with viral etiologies had seizure, refusal of feeds, involuntary movements, GCS < 8, dystonia, and focal deficit as predominant clinical features. Similar clinical features were also observed in viral encephalitis cases by Karmakar et al.[4] Vomiting and meningeal signs were more prominent in pyogenic cases whereas CNS TB presented with headache, raised ICP, cranial nerve palsy, and dystonia. Meningeal signs were conspicuously absent in cerebral malaria.
Only in about 60% of cases etiology could be ascertained. The probable reasons for such a big proportion of cases remaining etiologically inconclusive could be many, and includes: (i) specific serological and molecular-based investigations for many viral agents known to cause AFE in children (e.g., human herpesvirus 6, West Nile virus, etc.) were not done; (ii) many cases of bacterial meningitis may have received over the counter antibiotics making the CSF bacteriologically sterile and difficult to interpret, and latex agglutination test for pneumococcus, Haemophilus influenzae or Neisseria meningitidis was not done in such cases; (iii) nonavailability of investigations for autoimmune encephalitis (e.g. CSF anti-N-methyl-D-aspartate receptor antibody, Anti-voltage gated potassium channel antibody, etc.).[17] However, again, in the earlier study by Kumar et al., even with extensive investigations for viral etiology, 40% cases remained etiologically unexplained.[6] The above-mentioned investigations are also not routinely available in most of the secondary and tertiary level health-care facilities of India and other developing countries, where such patients are being managed.
Among the cases with an established cause, maximum was of viral etiology (23.3%) followed by pyogenic meningitis (14.4%) while malaria and CNS TB were found in only 8.9% cases each. The almost similar pattern of distribution of etiological diagnosis was in a recent study from central India.[3] Although the spectrum remains the same, the most common cause seem to vary according to the geographical region depending on the endemicity of different infective agents,[8] with viral causes being predominant in Asia,[8] cerebral malaria being the most common in Nepal and Africa[78] but bacterial meningitis in Pakistan and Papua New Guinea.[89] Among the cases with viral etiologies, dengue encephalopathy was the most common followed by H1N1 and HSV in this study. This is in contrast to the previous studies published from India, where Enterovirus,[4] JE virus or Adenovirus[6] were the predominant viral agents. This could be explained by the great increase in the incidence of dengue and H1N1 in the recent years in India, and the study population is belonging to a state with high burden for both the infections.[2425] Recent studies also indicate an increasing prevalence of dengue encephalopathy across the globe.[8] In synchrony with the previously described pediatric cases across the world, pneumococcus followed by H. influenzae was the most common bacteria isolated from pyogenic meningitis cases.[8]
The overall mortality rate of 40% observed in the study though much higher than recent studies from India[35] is comparable to the studies from other resource-poor settings, with reported mortality rates of 15%–58%.[8] The higher mortality rates could possibly be due to a higher number of sick patients in the present study, evidenced by the finding of a high number of children having GCS < 8, shock, and respiratory failure, which were also risk factors for mortality. Excluding cases with an inconclusive etiology, highest mortality was observed with viral cases (25%) followed by pyogenic meningitis (13.9%) while all the cases of enteric encephalopathy survived.
Various risk factors for death identified in the previous studies include low GCS, hypotension, raised ICP/intracranial herniation, breathing difficulties at presentation, bradycardia, severe anemia, and younger age while the association with seizure has been variable.[38] In the present study also GCS < 8, shock, raised ICP, and respiratory failure were identified as the risk factors associated with increased mortality.
Some limitations were noted and must be acknowledged in this study. The number of the study participants was not high, thereby limiting power of the analysis; being a tertiary care center based study, referral bias could not be ruled out; nonavailability of specific investigations for many viral etiologies of AFE, and autoimmune encephalitis; and the children were not followed up for long-term complications and sequelae.[826] Despite these limitations, assuming that the sample of patients studied is representative, the strength of the study was its robust methodology and prospective design. In a recent review of childhood acute nontraumatic coma,[8] the authors acknowledged that there is a significant paucity of knowledge on the etiology, pathophysiology, risk factors, outcomes, and appropriate interventions for childhood acute coma in resource-poor settings. We hope that our study would possibly contribute in filling the lacunae of our current understanding of AFE in developing countries.
CONCLUSION
AFE, though a rare diagnosis in children, is associated with significant morbidity and high mortality, particularly in a developing country like India. With nonavailability of many pathogen-specific microbiological investigations, etiological agent may remain elusive in a considerable proportion of cases. Nevertheless, many cases being viral in origin, where no specific treatment is available or highly effective, early institution of aggressive supportive care may be able to decrease mortality and long-term morbidity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Febrile encephalopathy: Challenges in management. J Assoc Physicians India. 2006;54:845-7.
- [Google Scholar]
- World Health Oraganisation. Acute Encephalitis Syndrome. Japanese Encephalitis Surveillance Standards. From WHO-Recommended Standards for Surveillance of Selected Vaccine-Preventable Diseases. WHO/V&B/03.01. 2006. Available from: http://www.apps.who.int/iris/bitstream/10665/68334/1/WHO_V.B_03.01_eng.pdf
- [Google Scholar]
- Acute febrile encephalopathy in children and predictors of mortality. J Clin Diagn Res. 2014;8:PC09-11.
- [Google Scholar]
- A study of acute febrile encephalopathy with special reference to viral etiology. Indian J Pediatr. 2008;75:801-5.
- [Google Scholar]
- Virological investigations of acute encephalopathy in India. Arch Dis Child. 1990;65:1227-30.
- [Google Scholar]
- Clinical and etiological profile of acute febrile encephalopathy in Eastern Nepal. Indian J Pediatr. 2009;76:1109-11.
- [Google Scholar]
- Childhood acute non-traumatic coma: Aetiology and challenges in management in resource-poor countries of Africa and Asia. Paediatr Int Child Health. 2013;33:129-38.
- [Google Scholar]
- The aetiology, clinical presentations and outcome of febrile encephalopathy in children in Papua new guinea. Ann Trop Paediatr. 2010;30:109-18.
- [Google Scholar]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. WHO/NMH/NHD/MNM/11.1. 2011. Geneva: World Health Organization; Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf
- [Google Scholar]
- Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- [Google Scholar]
- British Thoracic Society Standards of Care Committee. Non-invasive ventilation in acute respiratory failure. Thorax. 2002;57:192-211.
- [Google Scholar]
- Guidelines for the diagnosis and management of disseminated intravascular coagulation. British committee for standards in haematology. Br J Haematol. 2009;145:24-33.
- [Google Scholar]
- Modified Kuppuswamy's Socioeconomic Scale: Social researcher should include updated income criteria, 2012. Indian J Community Med. 2013;38:185-6.
- [Google Scholar]
- IAP growth monitoring guidelines for children from birth to 18 years. Indian Pediatr. 2007;44:187-97.
- [Google Scholar]
- Role of Glasgow coma scale in pediatric nontraumatic coma. Indian Pediatr. 2003;40:620-5.
- [Google Scholar]
- Tubercular meningitis in children: Clinical, pathological, and radiological profile and factors associated with mortality. J Neurosci Rural Pract. 2016;7:400-4.
- [Google Scholar]
- World Health Organization. Guidelines for the Treatment of Malaria. 2010. (2nd ed). Geneva: World Health Organization; Available from: http://www.ncbi.nlm.nih.gov/books/NBK254223/pdf/Bookshelf_NBK254223.pdf
- [Google Scholar]
- Clinical and public health aspects of tuberculous meningitis in children. J Pediatr. 1995;127:27-33.
- [Google Scholar]
- Causality in acute encephalitis: Defining aetiologies. Epidemiol Infect. 2010;138:783-800.
- [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Neurologic complications associated with novel influenza A (H1N1) virus infection in children-Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:773-8.
- [Google Scholar]
- Current status of dengue and Chikungunya in India. WHO South East Asia J Public Health. 2014;3:22-6.
- [Google Scholar]
- 2015 resurgence of influenza A (H1N1) 09: Smoldering pandemic in India? J Glob Infect Dis. 2015;7:56-9.
- [Google Scholar]
- Short and long-term outcomes in children with suspected acute encephalopathy. Brain Dev. 2016;38:731-7.
- [Google Scholar]






